Page 155 - Read Online
P. 155
Chen et al. Microstructures 2023;3:2023025 https://dx.doi.org/10.20517/microstructures.2023.12 Page 21 of 31
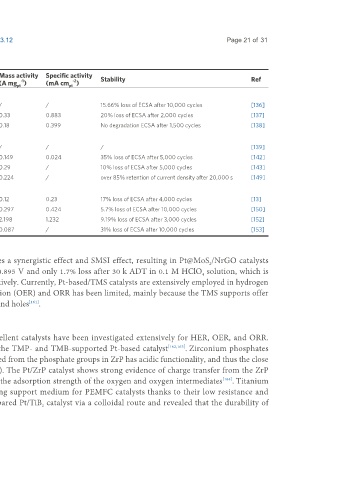
Table 4. ORR performance and stability of Carbide-based Pt catalysts
Support electrical conductivity ECSA Mass activity Specific activity
Catalyst Pt (wt.%) Size of Pt (nm) -1 2 -1 -1 -2 Stability Ref
(S cm ) (m g ) (A mg ) (mA cm pt )
pt
pt
TiC-based catalysts
-5
Pt/Ti C X 20 3-7 1.2 × 10 54.88 / / 15.66% loss of ECSA after 10,000 cycles [136]
3 2 2
Pt Pd-TiC-TiO 2 / 3.15 0.12 37.6 0.33 0.883 20% loss of ECSA after 2,000 cycles [137]
3
-2
Pt/Ti AlC 14.8 2 4.3 × 10 44.81 0.18 0.399 No degradation ECSA after 1,500 cycles [138]
3 2
M C-based catalysts
2
Pt-Mo C/CNTs 16 3-6 / / / / / [139]
2
Pt/Mo C-F / 3.25 / 58.5 0.149 0.024 35% loss of ECSA after 5,000 cycles [142]
2
Pt/M C 5 1 / / 0.29 / 10% loss of ECSA after 5,000 cycles [143]
2
Pt /Mo C 2.36 / / / 0.224 / over 85% retention of current density after 20,000 s [149]
quasi 2
Other carbide-based catalysts
ALD-Pt-ZrC 19 2-4 / / 0.12 0.23 17% loss of ECSA after 4,000 cycles [13]
Pt-Ta O TaC 4.95 2.4 / 70 0.297 0.424 5.7% loss of ECSA after 10,000 cycles [150]
2
5-
Pt-Ni/WC 9.425 4 / 178.4 2.198 1.232 9.19% loss of ECSA after 3,000 cycles [152]
Pt/NbC/C 30 3.1 10 52 0.087 / 31% loss of ECSA after 10,000 cycles [153]
Moreover, the attachment of MoS between NrGO and Pt NPs also generates a synergistic effect and SMSI effect, resulting in Pt@MoS /NrGO catalysts
2
2
exhibiting superior ORR activity and stability, with a half-wave potential at 0.895 V and only 1.7% loss after 30 k ADT in 0.1 M HClO solution, which is
4
greater than that of commercial Pt/C by a factor of 0.876 V and 3% loss, respectively. Currently, Pt-based/TMS catalysts are extensively employed in hydrogen
evolution reaction (HER), while the catalyst activity in oxygen evolution reaction (OER) and ORR has been limited, mainly because the TMS supports offer
poor reactivity, slow electronic conductivity, and fast reunion rate of electrons and holes .
[161]
Transition metal phosphide and boride
Transition metal phosphide and boride (TMP and TMB) themselves as excellent catalysts have been investigated extensively for HER, OER, and ORR.
However, there is still a lack of relevant work and understanding to design the TMP- and TMB-supported Pt-based catalyst [162,163] . Zirconium phosphates
(ZrP)-supported Pt NPs catalysts have recently been reported that ORR benefited from the phosphate groups in ZrP has acidic functionality, and thus the close
contact with Pt NPs can facilitate the active interface (as shown in Figure 9B). The Pt/ZrP catalyst shows strong evidence of charge transfer from the ZrP
support to Pt NPs, contributing to SMSI effect, and in turn directly affecting the adsorption strength of the oxygen and oxygen intermediates . Titanium
[164]
diboride (TiB ), as an electrically conducting ceramic material, is a promising support medium for PEMFC catalysts thanks to their low resistance and
2
considerable chemical stability. In previous work, Yin et al. successfully prepared Pt/TiB catalyst via a colloidal route and revealed that the durability of
2