Page 151 - Read Online
P. 151
Page 18 of 31 Chen et al. Microstructures 2023;3:2023025 https://dx.doi.org/10.20517/microstructures.2023.12
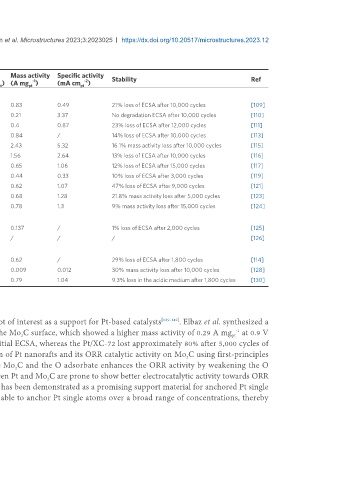
Table 3. ORR performance and stability of Nitrides-based Pt catalysts
Support electrical conductivity ECSA Mass activity Specific activity
Catalyst Pt (wt.%) Size of Pt (nm) -1 2 -1 -1 -2 Stability Ref
(S cm ) (m g ) (A mg ) (mA cm pt )
pt
pt
TiN-based catalysts
TiNiN@Pt 4.98 2-3 / 97 0.83 0.49 21% loss of ECSA after 10,000 cycles [109]
Pt/TiN NTs 20 3.65 118 61.3 0.21 3.37 No degradation ECSA after 10,000 cycles [110]
Pt/TiN NTs 20 3.75 85 45.8 0.4 0.87 23% loss of ECSA after 12,000 cycles [111]
Pt/Ti Co N 20 3.34 / 51.5 0.84 / 14% loss of ECSA after 10,000 cycles [113]
0.95 0.05
Pt Cu/TiN NTs 20 28 184 45.7 2.43 5.32 16.1% mass activity loss after 10,000 cycles [115]
3
Pt/Ti Cu N NFs 20 2.3 / 57.5 1.56 2.64 13% loss of ECSA after 10,000 cycles [116]
0.9 0.1
Pt/TiN NPs 20 3.0 679 / 0.65 1.06 12% loss of ECSA after 15,000 cycles [117]
TiN@Pt 12 2-3 / 66 0.44 0.33 10% loss of ECSA after 3,000 cycles [119]
Pt/Ti Mo N 20 3.4 / 54.9 0.62 1.07 47% loss of ECSA after 9,000 cycles [121]
0.8
0.2
Fe Pt/Ti Cr N 10.5 1-2 / 52.8 0.68 1.28 21.8% mass activity loss after 5,000 cycles [123]
3 0.5 0.5
Pt/Ti Ni N NTs 20 3.1 / 59.7 0.78 1.3 9% mass activity loss after 15,000 cycles [124]
0.9 0.1
VN-based catalysts
Pt-VN/GC 10 3.8 / 12.6 0.137 / 1% loss of ECSA after 2,000 cycles [125]
Pt/VN 15 2-8 / / / / / [126]
CrN-based catalysts
Pt/Ti Cr N NTs 20 3.0 / 52 0.62 / 29% loss of ECSA after 1,800 cycles [114]
0.95 0.05
Pt/CrN 20 3.9 69 75.3 0.009 0.012 30% mass activity loss after 10,000 cycles [128]
Pt/Ti Cr N /G 15.6 4 / 76.2 0.79 1.04 9.3% loss in the acidic medium after 1,800 cycles [130]
0.5
0.5
2
Molybdenum carbide (Mo C)
2
Molybdenum carbide (Mo C) is another carbide material that has received a lot of interest as a support for Pt-based catalysts [139-142] . Elbaz et al. synthesized a
2
Pt/Mo C catalyst with unique platinum rafts consisting of 6 atoms or less on the Mo C surface, which showed a higher mass activity of 0.29 A mg at 0.9 V
-1
pt
2
2
than Pt/XC-72 (0.19 A mg ). Meanwhile, the Pt/Mo C lost only 10% of its initial ECSA, whereas the Pt/XC-72 lost approximately 80% after 5,000 cycles of
-1
2
pt
accelerated durability testing . Subsequently, they investigated the formation of Pt nanorafts and its ORR catalytic activity on Mo C using first-principles
[143]
2
calculations and found that the O-O repulsion between the O atoms on the Mo C and the O adsorbate enhances the ORR activity by weakening the O
2
adsorption energy. Moreover, the SMSI effect and strong binding energy between Pt and Mo C are prone to show better electrocatalytic activity towards ORR
2
when compared to Pt/XC-72 (as shown in Figure 8C) . More recently, Mo C has been demonstrated as a promising support material for anchored Pt single
[144]
2
atoms, as the Mo atoms can provide SMSI with Pt species. Significantly, it is able to anchor Pt single atoms over a broad range of concentrations, thereby