Page 77 - Read Online
P. 77
Yang et al. Microstructures 2023;3:2023013 https://dx.doi.org/10.20517/microstructures.2022.30 Page 11 of 27
[64]
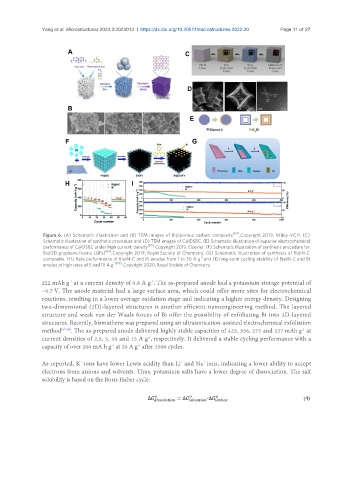
Figure 6. (A) Schematic illustration and (B) TEM images of Bi@porous carbon composite . Copyright 2019, Wiley-VCH. (C)
Schematic illustration of synthetic procedure and (D) TEM images of C@DSBC. (E) Schematic illustration of superior electrochemical
[60]
performance of C@DSBC under high current density . Copyright 2019, Elsevier. (F) Schematic illustration of synthesis procedure for
[63]
Bi@3D graphene foams (GFs) . Copyright 2019, Royal Society of Chemistry. (G) Schematic illustration of synthesis of Bi@N-C
-1
composite. (H) Rate performance of Bi@N-C and Bi anodes from 1 to 30 A g and (I) long-term cycling stability of Bi@N-C and Bi
-1 [65]
anodes at high rates of 5 and 10 A g . Copyright 2020, Royal Society of Chemistry.
222 mAh g at a current density of 0.8 A g . The as-prepared anode had a potassium storage potential of
-1
-1
~0.7 V. The anode material had a large surface area, which could offer more sites for electrochemical
reactions, resulting in a lower average oxidation stage and indicating a higher energy density. Designing
two-dimensional (2D)-layered structures is another efficient nanoengineering method. The layered
structure and weak van der Waals forces of Bi offer the possibility of exfoliating Bi into 2D-layered
structures. Recently, bismuthene was prepared using an ultrasonication-assisted electrochemical exfoliation
-1
method [67-69] . The as-prepared anode delivered highly stable capacities of 423, 356, 275 and 227 mAh g at
current densities of 2.5, 5, 10 and 15 A g , respectively. It delivered a stable cycling performance with a
-1
-1
-1
capacity of over 200 mA h g at 20 A g after 2500 cycles.
As reported, K ions have lower Lewis acidity than Li and Na ions, indicating a lower ability to accept
+
+
+
electrons from anions and solvents. Thus, potassium salts have a lower degree of dissociation. The salt
solubility is based on the Born-Haber cycle: