Page 72 - Read Online
P. 72
Yang et al. Microstructures 2023;3:2023013 https://dx.doi.org/10.20517/microstructures.2022.30 Page 7 of 27
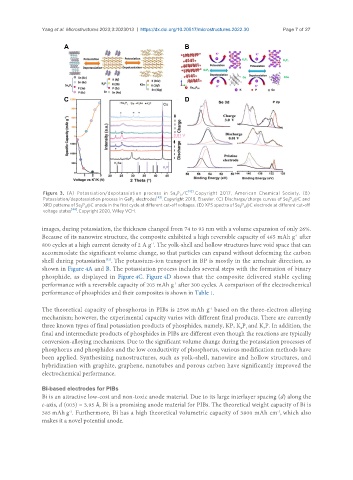
Figure 3. (A) Potassiation/depotassiation process in Sn P /C [42] . Copyright 2017, American Chemical Society. (B)
4 3
Potassiation/depotassiation process in GeP electrodes [43] . Copyright 2018, Elsevier. (C) Discharge/charge curves of Se P @C and
5
3 4
XRD patterns of Se P @C anode in the first cycle at different cut-off voltages. (D) XPS spectra of Se P @C electrode at different cut-off
3 4
3 4
voltage states [44] . Copyright 2020, Wiley VCH.
images, during potassiation, the thickness changed from 74 to 93 nm with a volume expansion of only 26%.
-1
Because of its nanowire structure, the composite exhibited a high reversible capacity of 465 mAh g after
800 cycles at a high current density of 2 A g . The yolk-shell and hollow structures have void space that can
-1
accommodate the significant volume change, so that particles can expand without deforming the carbon
shell during potassiation . The potassium-ion transport in BP is mostly in the armchair direction, as
[52]
shown in Figure 4A and B. The potassiation process includes several steps with the formation of binary
phosphide, as displayed in Figure 4C. Figure 4D shows that the composite delivered stable cycling
-1
performance with a reversible capacity of 205 mAh g after 300 cycles. A comparison of the electrochemical
performance of phosphides and their composites is shown in Table 1.
The theoretical capacity of phosphorus in PIBs is 2596 mAh g based on the three-electron alloying
-1
mechanism; however, the experimental capacity varies with different final products. There are currently
three known types of final potassiation products of phosphides, namely, KP, K P and K P. In addition, the
3
4 3
final and intermediate products of phosphides in PIBs are different even though the reactions are typically
conversion-alloying mechanisms. Due to the significant volume change during the potassiation processes of
phosphorus and phosphides and the low conductivity of phosphorus, various modification methods have
been applied. Synthesizing nanostructures, such as yolk-shell, nanowire and hollow structures, and
hybridization with graphite, graphene, nanotubes and porous carbon have significantly improved the
electrochemical performance.
Bi-based electrodes for PIBs
Bi is an attractive low-cost and non-toxic anode material. Due to its large interlayer spacing (d) along the
c-axis, d (003) = 3.95 Å, Bi is a promising anode material for PIBs. The theoretical weight capacity of Bi is
-3
-1
385 mAh g . Furthermore, Bi has a high theoretical volumetric capacity of 3800 mAh cm , which also
makes it a novel potential anode.