Page 82 - Read Online
P. 82
Yang et al. Microstructures 2023;3:2023013 https://dx.doi.org/10.20517/microstructures.2022.30 Page 15 of 27
+
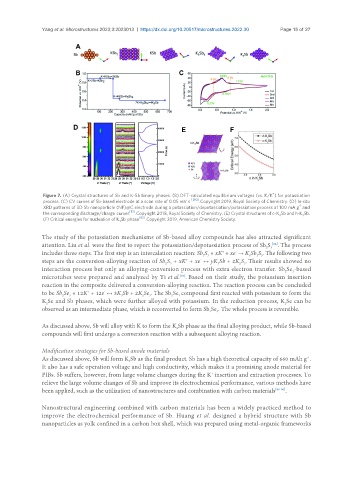
Figure 7. (A) Crystal structures of Sb and K-Sb binary phases. (B) DFT-calculated equilibrium voltages (vs. K/K ) for potassiation
process. (C) CV curves of Sb-based electrode at a scan rate of 0.05 mV s -1 [80] . Copyright 2019, Royal Society of Chemistry. (D) In-situ
-1
XRD patterns of 3D Sb nanoparticle (NP)@C electrode during a potassiation/depotassiation/potassiation process at 100 mA g and
the corresponding discharge/charge curves [81] . Copyright 2018, Royal Society of Chemistry. (E) Crystal structures of c-K Sb and h-K Sb.
3
3
(F) Critical energies for nucleation of K Sb phase [83] . Copyright 2019, American Chemistry Society.
3
The study of the potassiation mechanisms of Sb-based alloy compounds has also attracted significant
[84]
attention. Liu et al. were the first to report the potassiation/depotassiation process of Sb S . The process
2 3
includes three steps. The first step is an intercalation reaction: Sb S + xK + xe → K Sb S . The following two
+
-
3 3
2 3
x
steps are the conversion-alloying reaction of Sb S + xK + xe ↔ yK Sb + zK S . Their results showed no
+
-
3
2 3
2 3
interaction process but only an alloying-conversion process with extra electron transfer. Sb Se -based
3
2
microtubes were prepared and analyzed by Yi et al. . Based on their study, the potassium insertion
[85]
reaction in the composite delivered a conversion-alloying reaction. The reaction process can be concluded
-
+
to be Sb Se + 12K + 12e ↔ 3K Sb + 2K Se . The Sb Se compound first reacted with potassium to form the
3
3
3
2
2
3
2
K Se and Sb phases, which were further alloyed with potassium. In the reduction process, K Se can be
2
2
observed as an intermediate phase, which is reconverted to form Sb Se . The whole process is reversible.
3
2
As discussed above, Sb will alloy with K to form the K Sb phase as the final alloying product, while Sb-based
3
compounds will first undergo a conversion reaction with a subsequent alloying reaction.
Modification strategies for Sb-based anode materials
As discussed above, Sb will form K Sb as the final product. Sb has a high theoretical capacity of 660 mAh g .
-1
3
It also has a safe operation voltage and high conductivity, which makes it a promising anode material for
PIBs. Sb suffers, however, from large volume changes during the K insertion and extraction processes. To
+
relieve the large volume changes of Sb and improve its electrochemical performance, various methods have
been applied, such as the utilization of nanostructures and combination with carbon materials [86-90] .
Nanostructural engineering combined with carbon materials has been a widely practiced method to
improve the electrochemical performance of Sb. Huang et al. designed a hybrid structure with Sb
nanoparticles as yolk confined in a carbon box shell, which was prepared using metal-organic frameworks