Page 469 - Read Online
P. 469
Page 8 of 14 Saadi et al. Vessel Plus 2020;4:41 I http://dx.doi.org/10.20517/2574-1209.2020.54
A B C
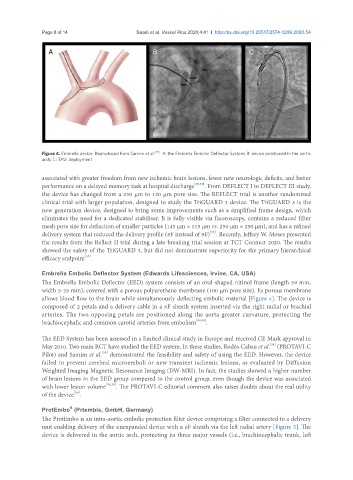
Figure 4. Embrella device. Reproduced from Samim et al. [35] . A: the Embrella Embolic Deflector System; B: device positioned in the aortic
arch; C: TAVI deployment
associated with greater freedom from new ischemic brain lesions, fewer new neurologic deficits, and better
performance on a delayed memory task at hospital discharge [30,31] . From DEFLECT I to DEFLECT III study,
the device has changed from a 250 μm to 130 μm pore size. The REFLECT trial is another randomized
clinical trial with larger population, designed to study the TriGUARD 3 device. The TriGUARD 3 is the
new generation device, designed to bring some improvements such as a simplified frame design, which
eliminates the need for a dedicated stabiliser. It is fully visible via fluoroscopy, contains a reduced filter
mesh pore size for deflection of smaller particles (145 µm × 115 µm vs. 250 µm × 250 µm), and has a refined
[32]
delivery system that reduced the delivery profile (8F instead of 9F) . Recently, Jeffrey W. Moses presented
the results from the Reflect II trial during a late-breaking trial session at TCT Connect 2020. The results
showed the safety of the TriGUARD 3, but did not demonstrate superiority for the primary hierarchical
efficacy endpoint .
[33]
Embrella Embolic Deflector System (Edwards Lifesciences, Irvine, CA, USA)
The Embrella Embolic Deflector (EED) system consists of an oval-shaped nitinol frame (length 59 mm,
width 5-25 mm), covered with a porous polyurethane membrane (100 μm pore size). Its porous membrane
allows blood flow to the brain while simultaneously deflecting embolic material [Figure 4]. The device is
composed of 2 petals and a delivery cable in a 6F sheath system inserted via the right radial or brachial
arteries. The two opposing petals are positioned along the aorta greater curvature, protecting the
brachiocephalic and common carotid arteries from embolism [34,35] .
The EED System has been assessed in a limited clinical study in Europe and received CE Mark approval in
[34]
May 2010. Two main RCT have studied the EED system. In these studies, Rodés-Cabau et al. (PROTAVI-C
Pilot) and Samim et al. demonstrated the feasibility and safety of using the EED. However, the device
[35]
failed to prevent cerebral microemboli or new transient ischemic lesions, as evaluated by Diffusion
Weighted Imaging Magnetic Resonance Imaging (DW-MRI). In fact, the studies showed a higher number
of brain lesions in the EED group compared to the control group, even though the device was associated
with lower lesion volume [34,35] . The PROTAVI-C editorial comment also raises doubts about the real utility
[36]
of the device .
R
ProtEmbo (Prtembis, GmbH, Germany)
The ProtEmbo is an intra-aortic embolic protection filter device comprising a filter connected to a delivery
unit enabling delivery of the unexpanded device with a 6F sheath via the left radial artery [Figure 5]. The
device is delivered in the aortic arch, protecting its three major vessels (i.e., brachiocephalic trunk, left