Page 464 - Read Online
P. 464
Saadi et al. Vessel Plus 2020;4:41 I http://dx.doi.org/10.20517/2574-1209.2020.54 Page 3 of 14
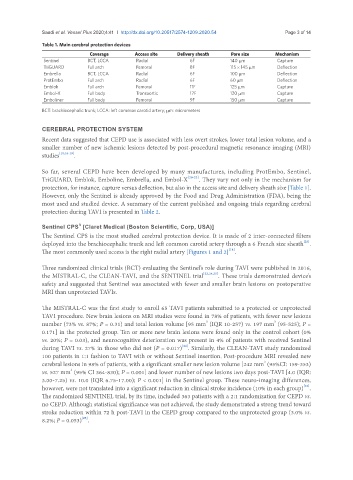
Table 1. Main cerebral protection devices
Coverage Access site Delivery sheath Pore size Mechanism
Sentinel BCT, LCCA Radial 6F 140 μm Capture
TriGUARD Full arch Femoral 8F 115 × 145 μm Deflection
Embrella BCT, LCCA Radial 6F 100 μm Deflection
ProtEmbo Full arch Radial 6F 60 μm Deflection
Emblok Full arch Femoral 11F 125 μm Capture
Embol-X Full body Transaortic 17F 120 μm Capture
Emboliner Full body Femoral 9F 150 μm Capture
BCT: brachiocephalic trunk; LCCA: left common carotid artery; μm: micrometers
CEREBRAL PROTECTION SYSTEM
Recent data suggested that CEPD use is associated with less overt strokes, lower total lesion volume, and a
smaller number of new ischemic lesions detected by post-procedural magnetic resonance imaging (MRI)
studies [10,16-19] .
So far, several CEPD have been developed by many manufactures, including ProtEmbo, Sentinel,
TriGUARD, Emblok, Emboline, Embrella, and Embol-X [20-22] . They vary not only in the mechanism for
protection, for instance, capture versus deflection, but also in the access site and delivery sheath size [Table 1].
However, only the Sentinel is already approved by the Food and Drug Administration (FDA), being the
most used and studied device. A summary of the current published and ongoing trials regarding cerebral
protection during TAVI is presented in Table 2.
R
Sentinel CPS [Claret Medical (Boston Scientific, Corp, USA)]
The Sentinel CPS is the most studied cerebral protection device. It is made of 2 inter-connected filters
[23]
deployed into the brachiocephalic trunk and left common carotid artery through a 6 French size sheath .
[18]
The most commonly used access is the right radial artery [Figures 1 and 2] .
Three randomized clinical trials (RCT) evaluating the Sentinel’s role during TAVI were published in 2016,
the MISTRAL-C, the CLEAN-TAVI, and the SENTINEL trial [22,24,25] . These trials demonstrated device’s
safety and suggested that Sentinel was associated with fewer and smaller brain lesions on postoperative
MRI than unprotected TAVIs.
The MISTRAL-C was the first study to enroll 65 TAVI patients submitted to a protected or unprotected
TAVI procedure. New brain lesions on MRI studies were found in 78% of patients, with fewer new lesions
3
3
number (73% vs. 87%; P = 0.31) and total lesion volume [95 mm (IQR 10-257) vs. 197 mm (95-525); P =
0.171] in the protected group. Ten or more new brain lesions were found only in the control cohort (0%
vs. 20%; P = 0.03), and neurocognitive deterioration was present in 4% of patients with received Sentinel
[24]
during TAVI vs. 27% in those who did not (P = 0.017) . Similarly, the CLEAN-TAVI study randomized
100 patients in 1:1 fashion to TAVI with or without Sentinel insertion. Post-procedure MRI revealed new
3
cerebral lesions in 98% of patients, with a significant smaller new lesion volume [242 mm (95%CI: 159-353)
3
vs. 527 mm (95% CI 364-830); P = 0.001] and lower number of new lesions two days post-TAVI [4.0 (IQR:
3.00-7.25) vs. 10.0 (IQR 6.75-17.00); P < 0.001] in the Sentinel group. These neuro-imaging differences,
[22]
however, were not translated into a significant reduction in clinical stroke incidence (10% in each group) .
The randomized SENTINEL trial, by its time, included 363 patients with a 2:1 randomization for CEPD vs.
no CEPD. Although statistical significance was not achieved, the study demonstrated a strong trend toward
stroke reduction within 72 h post-TAVI in the CEPD group compared to the unprotected group (3.0% vs.
[25]
8.2%; P = 0.053) .