Page 465 - Read Online
P. 465
Page 4 of 14 Saadi et al. Vessel Plus 2020;4:41 I http://dx.doi.org/10.20517/2574-1209.2020.54
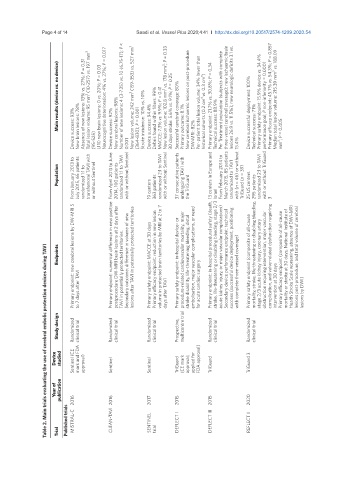
Main results (device vs. no device) Device success: 93% New brain lesions: 78% Absence of new lesions: 13% vs. 27%; P = 0.31 Total lesion volume: 95 mm 3 (10-257) vs. 197 mm 3 ≥ 10 new brain lesions: 0 vs. 20%; P = 0.03 Neurocognitive deterioration: 4% vs. 27%; P = 0.017 Device success: 92% New cerebral lesions: 98% Number of new lesions: 4 (3-7.25) vs. 10 (6.75-17); P < New lesion volume: 242 mm 3 (159-353) vs. 527 mm 3 (364-830); P = 0.00
Population From January 2013 to July 2015, 65 patients randomized 1:1 to transfemoral TAVI with (95-525) or without Sentinel From April 2013 to June 2014, 100 patients randomized 1:1 to TAVI 0.001 with or without Sentinel 19 centers 363 patients randomized 2:1 to TAVI with or without Sentinel 37 consecutive patients undergoing TAVI with the TriGuard Primary endpoint: in-hospital procedural safety (death, 13 centers in Euro
Table 2. Main trials evaluating the use of cerebral embolic protection devices during TAVI
Endpoints Primary endpoint: new cerebral lesions by DW-MRI 5 Primary endpoint: numerical difference in new positive postprocedure DW-MRI brain lesions at 2 days after TAVI in potentially protected territories. Secondary outcome: difference in volume of new lesions after TAVI in potentially protected territories Primary safety endpoint: MACCE at 30 days Primary efficacy endpoint: reduction in new lesion volume in protected brain territories by
to 7 days after TAVI days after TAVI for acute cardiac surgery intervention at 30 days lesions by DWI)
Study design Randomized clinical trial Randomized clinical trial Randomized clinical trial Prospective, multicentre trial Randomized clinical trial Randomized clinical trial
Device studied Sentinel (CE mark and FDA approval) Sentinel Sentinel TriGuard (CE mark approval; applied for FDA approval) TriGuard TriGuard 3
Year of publication 2016 2016 2017 2015 2015 2020
Published trials MISTRAL-C CLEAN-TAVI SENTINEL DEFLECT I DEFLECT III REFLECT II
Trial trial