Page 470 - Read Online
P. 470
Saadi et al. Vessel Plus 2020;4:41 I http://dx.doi.org/10.20517/2574-1209.2020.54 Page 9 of 14
Figure 5. Illustration of ProtEmbo device
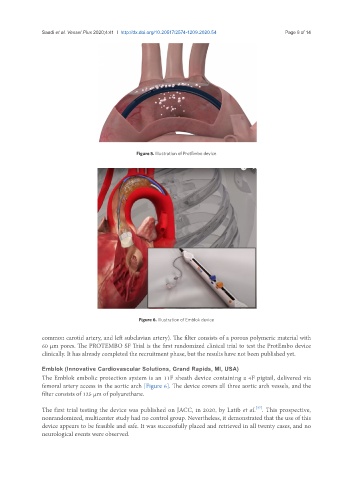
Figure 6. Illustration of Emblok device
common carotid artery, and left subclavian artery). The filter consists of a porous polymeric material with
60 μm pores. The PROTEMBO SF Trial is the first randomized clinical trial to test the ProtEmbo device
clinically. It has already completed the recruitment phase, but the results have not been published yet.
Emblok (Innovative Cardiovascular Solutions, Grand Rapids, MI, USA)
The Emblok embolic protection system is an 11F sheath device containing a 4F pigtail, delivered via
femoral artery access in the aortic arch [Figure 6]. The device covers all three aortic arch vessels, and the
filter consists of 125 μm of polyurethane.
The first trial testing the device was published on JACC, in 2020, by Latib et al. . This prospective,
[37]
nonrandomized, multicenter study had no control group. Nevertheless, it demonstrated that the use of this
device appears to be feasible and safe. It was successfully placed and retrieved in all twenty cases, and no
neurological events were observed.