Page 471 - Read Online
P. 471
Page 10 of 14 Saadi et al. Vessel Plus 2020;4:41 I http://dx.doi.org/10.20517/2574-1209.2020.54
A B
C
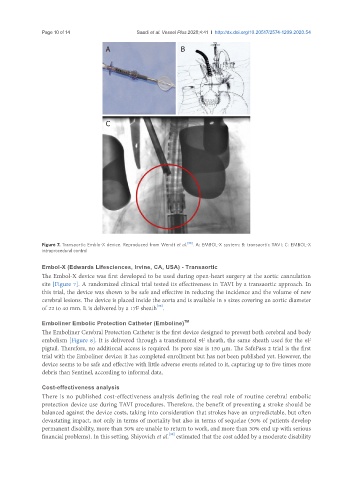
Figure 7. Transaortic Emblo-X device. Reproduced from Wendt et al. [38] . A: EMBOL-X system; B: transaortic TAVI; C: EMBOL-X
intraprocedural control
Embol-X (Edwards Lifesciences, Irvine, CA, USA) - Transaortic
The Embol-X device was first developed to be used during open-heart surgery at the aortic cannulation
site [Figure 7]. A randomized clinical trial tested its effectiveness in TAVI by a transaortic approach. In
this trial, the device was shown to be safe and effective in reducing the incidence and the volume of new
cerebral lesions. The device is placed inside the aorta and is available in 5 sizes covering an aortic diameter
[38]
of 22 to 40 mm. It is delivered by a 17F sheath .
Emboliner Embolic Protection Catheter (Emboline) TM
The Emboliner Cerebral Protection Catheter is the first device designed to prevent both cerebral and body
embolism [Figure 8]. It is delivered through a transfemoral 9F sheath, the same sheath used for the 6F
pigtail. Therefore, no additional access is required. Its pore size is 150 μm. The SafePass 2 trial is the first
trial with the Emboliner device; it has completed enrollment but has not been published yet. However, the
device seems to be safe and effective with little adverse events related to it, capturing up to five times more
debris than Sentinel, according to informal data.
Cost-effectiveness analysis
There is no published cost-effectiveness analysis defining the real role of routine cerebral embolic
protection device use during TAVI procedures. Therefore, the benefit of preventing a stroke should be
balanced against the device costs, taking into consideration that strokes have an unpredictable, but often
devastating impact, not only in terms of mortality but also in terms of sequelae (50% of patients develop
permanent disability, more than 50% are unable to return to work, and more than 30% end up with serious
[39]
financial problems). In this setting, Shiyovich et al. estimated that the cost added by a moderate disability