Page 13 - Read Online
P. 13
Dong et al. UPI peptide as cancer therapeutic
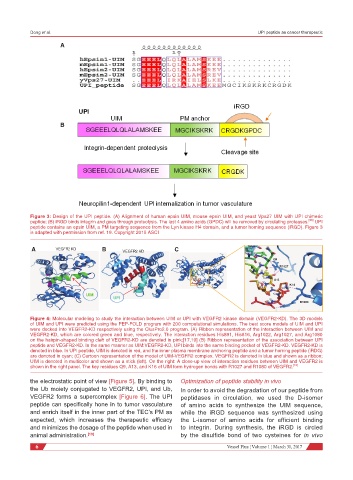
A
B
Figure 3: Design of the UPI peptide. (A) Alignment of human epsin UIM, mouse epsin UIM, and yeast Vps27 UIM with UPI chimeric
peptide; (B) iRGD binds integrin and goes through proteolysis. The last 4 amino acids (GPDC) will be removed by circulating proteases. [23] UPI
peptide contains an epsin UIM, a PM targeting sequence from the Lyn kinase H4 domain, and a tumor homing sequence (iRGD). Figure 3
is adapted with permission from ref. 19. Copyright 2015 ASCI
A VEGFR2 KD B VEGFR2 KD C
Q9
R1027
A13
K16
UIM
UPI
R1080
Figure 4: Molecular modeling to study the interaction between UIM or UPI with VEGFR2 kinase domain (VEGFR2-KD). The 3D models
of UIM and UPI were predicted using the PEP-FOLD program with 200 computational simulations. The best score models of UIM and UPI
were docked into VEGFR2-KD respectively using the ClusPro2.0 program. (A) Ribbon representation of the interaction between UIM and
VEGFR2-KD, which are colored green and blue, respectively. The interaction residues His891, His816, Arg1022, Arg1027, and Arg1080
on the hairpin-shaped binding cleft of VEGFR2-KD are denoted in pink;[17,19] (B) Ribbon representation of the association between UPI
peptide and VEGFR2-KD. In the same manner as UIM:VEGFR2-KD, UPI binds into the same binding pocket of VEGFR2-KD. VEGFR2-KD is
denoted in blue. In UPI peptide, UIM is denoted in red, and the inner plasma membrane anchoring peptide and a tumor homing peptide (iRDG)
are denoted in cyan; (C) Cartoon representation of the model of UIM-VEGFR2 complex. VEGFR2 is denoted in blue and shown as a ribbon;
UIM is denoted in multicolor and shown as a stick (left). On the right: A close-up view of interaction residues between UIM and VEGFR2 is
shown in the right panel. The key residues Q9, A13, and K16 of UIM form hydrogen bonds with R1027 and R1080 of VEGFR2. [19]
the electrostatic point of view [Figure 5]. By binding to Optimization of peptide stability in vivo
the Ub moiety conjugated to VEGFR2, UPI, and Ub, In order to avoid the degradation of our peptide from
VEGFR2 forms a supercomplex [Figure 6]. The UPI peptidases in circulation, we used the D-isomer
peptide can specifically hone in to tumor vasculature of amino acids to synthesize the UIM sequence,
and enrich itself in the inner part of the TEC’s PM as while the iRGD sequence was synthesized using
expected, which increases the therapeutic efficacy the L-isomer of amino acids for efficient binding
and minimizes the dosage of the peptide when used in to integrin. During synthesis, the iRGD is circled
animal administration. [19] by the disulfide bond of two cysteines for in vivo
6 Vessel Plus ¦ Volume 1 ¦ March 31, 2017