Page 35 - Read Online
P. 35
Page 6 of 28 Zhang et al. Soft Sci 2024;4:39 https://dx.doi.org/10.20517/ss.2024.34
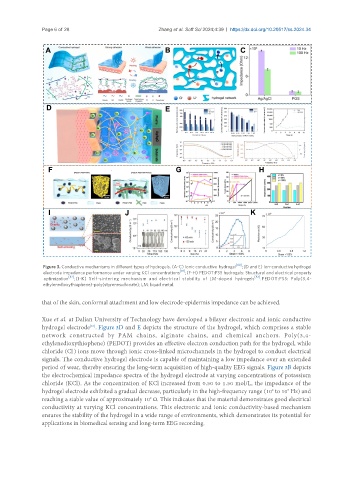
Figure 3. Conductive mechanisms in different types of hydrogels. (A-C) Ionic conductive hydrogel [80] ; (D and E) Ion-conductive hydrogel
electrode impedance performance under varying KCl concentrations [81] ; (F-H) PEDOT:PSS hydrogels: Structural and electrical property
optimization [82] ; (I-K) Self-sintering mechanism and electrical stability of LM-doped hydrogels [83] . PEDOT:PSS: Poly(3,4-
ethylenedioxythiophene)-poly(styrenesulfonate); LM: liquid metal.
that of the skin, conformal attachment and low electrode-epidermis impedance can be achieved.
Xue et al. at Dalian University of Technology have developed a bilayer electronic and ionic conductive
hydrogel electrode . Figure 3D and E depicts the structure of the hydrogel, which comprises a stable
[81]
network constructed by PAM chains, alginate chains, and chemical anchors. Poly(3,4-
ethylenedioxythiophene) (PEDOT) provides an effective electron conduction path for the hydrogel, while
chloride (Cl ) ions move through ionic cross-linked microchannels in the hydrogel to conduct electrical
-
signals. The conductive hydrogel electrode is capable of maintaining a low impedance over an extended
period of wear, thereby ensuring the long-term acquisition of high-quality EEG signals. Figure 3B depicts
the electrochemical impedance spectra of the hydrogel electrode at varying concentrations of potassium
chloride (KCl). As the concentration of KCl increased from 0.50 to 1.50 mol/L, the impedance of the
hydrogel electrode exhibited a gradual decrease, particularly in the high-frequency range (10 to 10 Hz) and
2
5
reaching a stable value of approximately 10 Ω. This indicates that the material demonstrates good electrical
2
conductivity at varying KCl concentrations. This electronic and ionic conductivity-based mechanism
ensures the stability of the hydrogel in a wide range of environments, which demonstrates its potential for
applications in biomedical sensing and long-term EEG recording.