Page 94 - Read Online
P. 94
Tang et al. Soft Sci. 2025, 5, 11 https://dx.doi.org/10.20517/ss.2024.62 Page 5 of 21
[40]
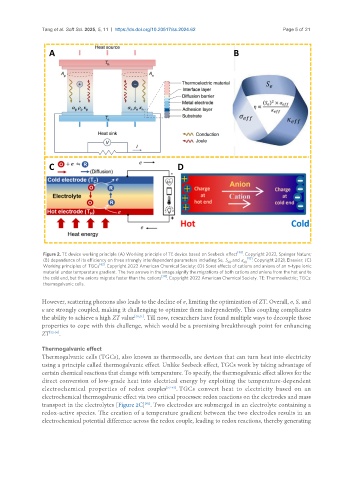
Figure 2. TE device working principle (A) Working principle of TE device based on Seebeck effect . Copyright 2022, Springer Nature;
[42]
(B) dependence of its efficiency on three strongly interdependent parameters including Se, S , and κ . Copyright 2021, Elsevier; (C)
eff eff
[62]
Working principles of TGCs . Copyright 2022 American Chemical Society; (D) Soret effects of cations and anions of an n-type ionic
material under temperature gradient. The two arrows in the image signify the migrations of both cations and anions from the hot end to
[70]
the cold end, but the anions migrate faster than the cations . Copyright 2022 American Chemical Society. TE: Thermoelectric; TGCs:
thermogalvanic cells.
However, scattering phonons also leads to the decline of σ, limiting the optimization of ZT. Overall, σ, S, and
κ are strongly coupled, making it challenging to optimize them independently. This coupling complicates
the ability to achieve a high ZT value [50,51] . Till now, researchers have found multiple ways to decouple those
properties to cope with this challenge, which would be a promising breakthrough point for enhancing
ZT [52-56] .
Thermogalvanic effect
Thermogalvanic cells (TGCs), also known as thermocells, are devices that can turn heat into electricity
using a principle called thermogalvanic effect. Unlike Seebeck effect, TGCs work by taking advantage of
certain chemical reactions that change with temperature. To specify, the thermogalvanic effect allows for the
direct conversion of low-grade heat into electrical energy by exploiting the temperature-dependent
electrochemical properties of redox couples [57-61] . TGCs convert heat to electricity based on an
electrochemical thermogalvanic effect via two critical processes: redox reactions on the electrodes and mass
transport in the electrolytes [Figure 2C] . Two electrodes are submerged in an electrolyte containing a
[62]
redox-active species. The creation of a temperature gradient between the two electrodes results in an
electrochemical potential difference across the redox couple, leading to redox reactions, thereby generating