Page 80 - Read Online
P. 80
Page 4 of 31 Kim et al. Soft Sci 2024;4:33 https://dx.doi.org/10.20517/ss.2024.28
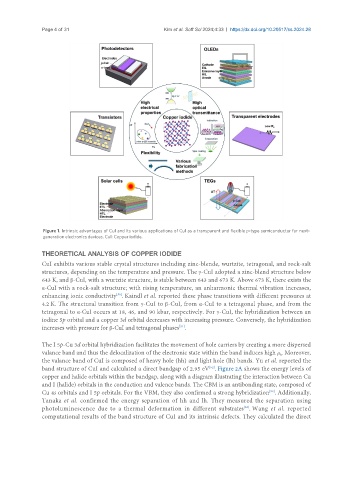
Figure 1. Intrinsic advantages of CuI and its various applications of CuI as a transparent and flexible p-type semiconductor for next-
generation electronics devices. CuI: Copper iodide.
THEORETICAL ANALYSIS OF COPPER IODIDE
CuI exhibits various stable crystal structures including zinc-blende, wurtzite, tetragonal, and rock-salt
structures, depending on the temperature and pressure. The γ-CuI adopted a zinc-blend structure below
643 K, and β-CuI, with a wurtzite structure, is stable between 643 and 673 K. Above 673 K, there exists the
α-CuI with a rock-salt structure; with rising temperature, an anharmonic thermal vibration increases,
enhancing ionic conductivity . Kaindl et al. reported these phase transitions with different pressures at
[50]
4.2 K. The structural transition from γ-CuI to β-CuI, from α-CuI to a tetragonal phase, and from the
tetragonal to α-CuI occurs at 18, 46, and 90 kbar, respectively. For γ-CuI, the hybridization between an
iodine 5p orbital and a copper 3d orbital decreases with increasing pressure. Conversely, the hybridization
[51]
increases with pressure for β-CuI and tetragonal phases .
The I 5p-Cu 3d orbital hybridization facilitates the movement of hole carriers by creating a more dispersed
valance band and thus the delocalization of the electronic state within the band induces high μ . Moreover,
h
the valance band of CuI is composed of heavy hole (hh) and light hole (lh) bands. Yu et al. reported the
[52]
band structure of CuI and calculated a direct bandgap of 2.95 eV . Figure 2A shows the energy levels of
copper and halide orbitals within the bandgap, along with a diagram illustrating the interaction between Cu
and I (halide) orbitals in the conduction and valence bands. The CBM is an antibonding state, composed of
Cu 4s orbitals and I 5p orbitals. For the VBM, they also confirmed a strong hybridization . Additionally,
[52]
Tanaka et al. confirmed the energy separation of hh and lh. They measured the separation using
[53]
photoluminescence due to a thermal deformation in different substrates . Wang et al. reported
computational results of the band structure of CuI and its intrinsic defects. They calculated the direct