Page 81 - Read Online
P. 81
Kim et al. Soft Sci 2024;4:33 https://dx.doi.org/10.20517/ss.2024.28 Page 5 of 31
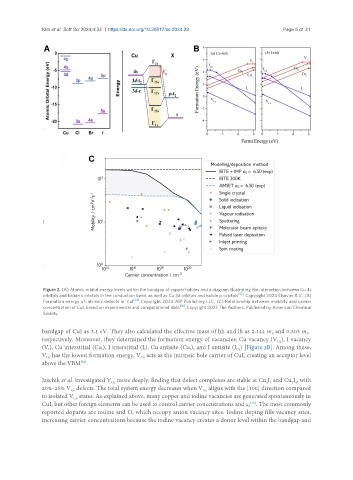
Figure 2. (A) Atomic orbital energy levels within the bandgap of copper halides and a diagram illustrating the interaction between Cu 4s
orbitals and halide s orbitals in the conduction band, as well as Cu 3d orbitals and halide p orbitals [52] . Copyright 2024 Elsevier B.V.; (B)
Formation energy of intrinsic defects in CuI [54] . Copyright 2024 AIP Publishing LLC; (C) Relationship between mobility and carrier
concentration of CuI, based on experimental and computational data [14] . Copyright 2023 The Authors. Published by American Chemical
Society.
bandgap of CuI as 3.1 eV. They also calculated the effective mass of hh and lh as 2.144 m and 0.303 m ,
0
0
respectively. Moreover, they determined the formation energy of vacancies: Cu vacancy (V ), I vacancy
Cu
(V ), Cu interstitial (Cu), I interstitial (I), Cu antisite (Cu ), and I antisite (I ) [Figure 2B]. Among these,
I
i
I
Cu
i
V has the lowest formation energy. V acts as the intrinsic hole carrier of CuI, creating an acceptor level
Cu
Cu
above the VBM .
[54]
Jaschik et al. investigated V more deeply, finding that defect complexes are stable at Cu I and Cu I , with
4 5
3 4
Cu
20%-25% V defects. The total system energy decreases when V aligns with the [100] direction compared
Cu
Cu
to isolated V states. As explained above, many copper and iodine vacancies are generated spontaneously in
Cu
CuI, but other foreign elements can be used to control carrier concentrations and μ . The most commonly
[55]
h
reported dopants are iodine and O, which occupy anion vacancy sites. Iodine doping fills vacancy sites,
increasing carrier concentrations because the iodine vacancy creates a donor level within the bandgap and