Page 18 - Read Online
P. 18
Page 6 of 14 Jo et al. Soft Sci 2024;4:27 https://dx.doi.org/10.20517/ss.2024.19
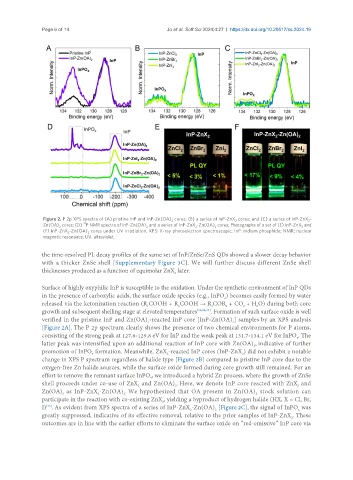
Figure 2. P 2p XPS spectra of (A) pristine InP and InP-Zn(OA) cores; (B) a series of InP-ZnX cores; and (C) a series of InP-ZnX -
2 2 2
31
Zn(OA) cores; (D) P NMR spectra of InP-Zn(OA) and a series of InP-ZnX -Zn(OA) cores; Photographs of a set of (E) InP-ZnX and
2 2 2 2 2
(F) InP-ZnX -Zn(OA) cores under UV irradiation. XPS: X-ray photoelectron spectroscopic; InP: indium phosphide; NMR: nuclear
2 2
magnetic resonance; UV: ultraviolet.
the time-resolved PL decay profiles of the same set of InP/ZnSe/ZnS QDs showed a slower decay behavior
with a thicker ZnSe shell [Supplementary Figure 3C]. We will further discuss different ZnSe shell
thicknesses produced as a function of equimolar ZnX later.
2
Surface of highly oxyphilic InP is susceptible to the oxidation. Under the synthetic environment of InP QDs
in the presence of carboxylic acids, the surface oxide species (e.g., InPO ) becomes easily formed by water
x
released via the ketonization reaction (R COOH + R COOH → R COR + CO + H O) during both core
2
1
2
2
1
2
growth and subsequent shelling stage at elevated temperatures [32,36,44] . Formation of such surface oxide is well
verified in the pristine InP and Zn(OA) -reacted InP core [InP-Zn(OA) ] samples by an XPS analysis
2
2
[Figure 2A]. The P 2p spectrum clearly shows the presence of two chemical environments for P atoms,
consisting of the strong peak at 127.6-129.8 eV for InP and the weak peak at 131.7-134.1 eV for InPO . The
x
latter peak was intensified upon an additional reaction of InP core with Zn(OA) , indicative of further
2
promotion of InPO formation. Meanwhile, ZnX -reacted InP cores (InP-ZnX ) did not exhibit a notable
2
2
x
change in XPS P spectrum regardless of halide type [Figure 2B] compared to pristine InP core due to the
oxygen-free Zn halide sources, while the surface oxide formed during core growth still remained. For an
effort to remove the remnant surface InPO , we introduced a hybrid Zn process, where the growth of ZnSe
4
shell proceeds under co-use of ZnX and Zn(OA) . Here, we denote InP core reacted with ZnX and
2
2
2
Zn(OA) as InP-ZnX -Zn(OA) . We hypothesized that OA present in Zn(OA) stock solution can
2
2
2
2
participate in the reaction with co-existing ZnX , yielding a byproduct of hydrogen halide (HX, X = Cl, Br,
2
I) . As evident from XPS spectra of a series of InP-ZnX -Zn(OA) [Figure 2C], the signal of InPO was
[45]
2
2
x
greatly suppressed, indicative of its effective removal, relative to the prior samples of InP-ZnX . These
2
outcomes are in line with the earlier efforts to eliminate the surface oxide on “red-emissive” InP core via