Page 91 - Read Online
P. 91
Kim et al. Soft Sci 2024;4:24 https://dx.doi.org/10.20517/ss.2024.09 Page 17 of 27
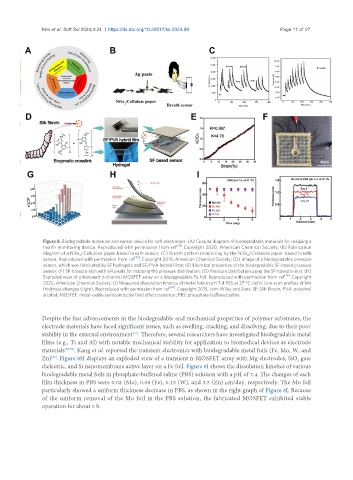
Figure 8. Biodegradable materials and sensor device for soft electronics. (A) Circular diagram of biodegradable materials for realizing a
health monitoring device. Reproduced with permission from ref [74] . Copyright 2020, American Chemical Society; (B) Fabrication
diagram of a NiSe /Cellulose paper-based breath sensor; (C) Breath pattern monitoring by the NiSe /Cellulose paper-based breath
2
2
sensor. Reproduced with permission from ref [75] . Copyright 2019, American Chemical Society; (D) Image of a biodegradable pressure
sensor, which was fabricated by SF hydrogels and SF/PVA hybrid films; (E) Electrical properties of the biodegradable SF-based pressure
sensor; (F) SF-based e-skin with 64 pixels for mapping the pressure distribution; (G) Pressure distribution using the SF-based e-skin; (H)
Exploded view of a transient n-channel MOSFET array on a biodegradable Fe foil. Reproduced with permission from ref [76] . Copyright
2022, American Chemical Society; (I) Measured dissolution kinetics of metal foils in pH 7.4 PBS at 37 °C (left). Line scan profiles of Mo
thickness changes (right). Reproduced with permission from ref [80] . Copyright 2015, John Wiley and Sons. SF: Silk fibroin; PVA: polyvinyl
alcohol; MOSFET: metal-oxide-semiconductor field effect transistor; PBS: phosphate-buffered saline.
Despite the fast advancements in the biodegradable and mechanical properties of polymer substrates, the
electrode materials have faced significant issues, such as swelling, cracking, and dissolving, due to their poor
stability in the external environment . Therefore, several researchers have investigated biodegradable metal
[77]
films (e.g., Ti and Al) with notable mechanical stability for application to biomedical devices as electrode
materials [78,79] . Kang et al. reported the transient electronics with biodegradable metal foils (Fe, Mo, W, and
Zn) . Figure 8H displays an exploded view of a transient n-MOSFET array with Mg electrodes, SiO gate
[80]
2
dielectric, and Si nanomembrane active layer on a Fe foil. Figure 8I shows the dissolution kinetics of various
biodegradable metal foils in phosphate-buffered saline (PBS) solution with a pH of 7.4. The changes of each
film thickness in PBS were 0.02 (Mo), 0.08 (Fe), 0.15 (W), and 3.5 (Zn) µm/day, respectively. The Mo foil
particularly showed a uniform thickness decrease in PBS, as shown in the right graph of Figure 8I. Because
of the uniform removal of the Mo foil in the PBS solution, the fabricated MOSFET exhibited stable
operation for about 5 h.