Page 42 - Read Online
P. 42
Page 8 of 34 Bai et al. Soft Sci 2023;3:40 https://dx.doi.org/10.20517/ss.2023.38
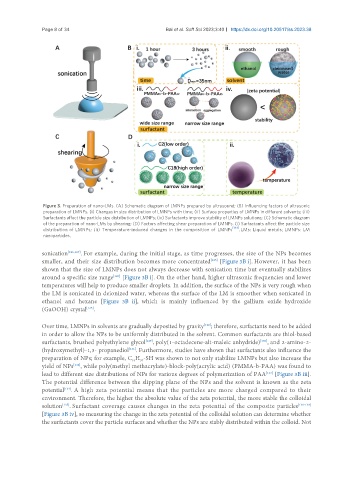
Figure 3. Preparation of nano-LMs. (A) Schematic diagram of LMNPs prepared by ultrasound; (B) Influencing factors of ultrasonic
preparation of LMNPs. (i) Changes in size distribution of LMNPs with time; (ii) Surface properties of LMNPs in different solvents; (iii)
Surfactants affect the particle size distribution of LMNPs; (iv) Surfactants improve stability of LMNPs solutions; (C) Schematic diagram
of the preparation of nano-LMs by shearing; (D) Factors affecting shear preparation of LMNPs. (i) Surfactants affect the particle size
distribution of LMNPs; (ii) Temperature-induced changes in the composition of LMNPs [144] . LMs: Liquid metals; LMNPs: LM
nanoparticles.
sonication [126,127] . For example, during the initial stage, as time progresses, the size of the NPs becomes
smaller, and their size distribution becomes more concentrated [Figure 3B i]. However, it has been
[125]
shown that the size of LMNPs does not always decrease with sonication time but eventually stabilizes
around a specific size range [Figure 3B i]. On the other hand, higher ultrasonic frequencies and lower
[126]
temperatures will help to produce smaller droplets. In addition, the surface of the NPs is very rough when
the LM is sonicated in deionized water, whereas the surface of the LM is smoother when sonicated in
ethanol and hexane [Figure 3B ii], which is mainly influenced by the gallium oxide hydroxide
(GaOOH) crystal .
[125]
Over time, LMNPs in solvents are gradually deposited by gravity ; therefore, surfactants need to be added
[128]
in order to allow the NPs to be uniformly distributed in the solvent. Common surfactants are thiol-based
surfactants, brushed polyethylene glycol , poly(1-octadecene-alt-maleic anhydride) , and 2-amino-2-
[129]
[130]
[131]
(hydroxymethyl)-1,3- propanediol . Furthermore, studies have shown that surfactants also influence the
preparation of NPs; for example, C H -SH was shown to not only stabilize LMNPs but also increase the
18
37
[132]
yield of NPs , while poly(methyl methacrylate)-block-poly(acrylic acid) (PMMA-b-PAA) was found to
lead to different size distributions of NPs for various degrees of polymerization of PAA [Figure 3B iii].
[133]
The potential difference between the slipping plane of the NPs and the solvent is known as the zeta
potential . A high zeta potential means that the particles are more charged compared to their
[134]
environment. Therefore, the higher the absolute value of the zeta potential, the more stable the colloidal
solution . Surfactant coverage causes changes in the zeta potential of the composite particles [136-138]
[135]
[Figure 3B iv], so measuring the change in the zeta potential of the colloidal solution can determine whether
the surfactants cover the particle surfaces and whether the NPs are stably distributed within the colloid. Not