Page 38 - Read Online
P. 38
Page 4 of 34 Bai et al. Soft Sci 2023;3:40 https://dx.doi.org/10.20517/ss.2023.38
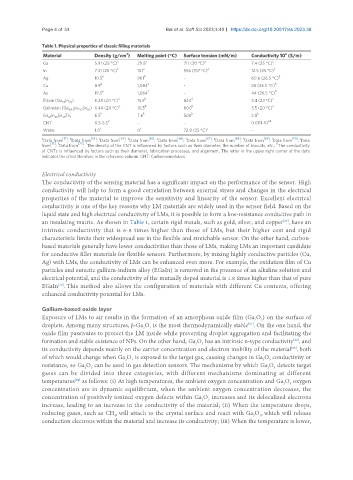
Table 1. Physical properties of classic filling materials
3
6
Material Density (g/cm ) Melting point (°C) Surface tension (mN/m) Conductivity 10 (S/m)
Ga 5.91 (25 °C) c 29.8 c 711 (30 °C) a 7.4 (25 °C) c
c c a c
In 7.31 (25 °C) 157 556 (157 °C) 12.5 (25 °C)
e h b
Ag 10.5 961 - 60.6 (26.5 °C)
g a b
Cu 8.9 1,084 - 58 (26.5 °C)
e f b
Au 19.3 1,064 - 44 (26.5 °C)
EGaIn (Ga In ) 6.28 (20 °C) c 15.5 b 624 d 3.4 (22 °C) c
75
25
c b b c
Galinstan (Ga 68.5 In 21.5 Sn ) 6.44 (20 °C) 10.5 600 3.5 (20 °C)
10
Ga In Sn Zn 1 6.5 k 7.6 k 500 k 2.8 k
61
13
25
i,* i,#
CNT 0.5-3.5 - - 0.001-10
Water 1.0 j 0 j 72.8 (25 °C) j -
a [51] b [63] c [64] d [65] e [66] f [67] g [68] h [69] i [70] j
Data from ; Data from ; Data from ; Data from ; Data from ; Data from ; Data from ; Data from ; Data from ; Data
[71] k [72] * #
from ; Data from ; The density of the CNT is influenced by factors such as their diameter, the number of biscuits, etc.; The conductivity
of CNTs is influenced by factors such as their diameter, fabrication processes, and alignment. The letter in the upper right corner of the data
indicates the cited literature in the reference column. CNT: Carbon nanotubes.
Electrical conductivity
The conductivity of the sensing material has a significant impact on the performance of the sensor. High
conductivity will help to form a good correlation between external stress and changes in the electrical
properties of the material to improve the sensitivity and linearity of the sensor. Excellent electrical
conductivity is one of the key reasons why LM materials are widely used in the sensor field. Based on the
liquid state and high electrical conductivity of LMs, it is possible to form a low-resistance conductive path in
an insulating matrix. As shown in Table 1, certain rigid metals, such as gold, silver, and copper , have an
[63]
intrinsic conductivity that is 6-8 times higher than those of LMs, but their higher cost and rigid
characteristic limits their widespread use in the flexible and stretchable sensor. On the other hand, carbon-
based materials generally have lower conductivities than those of LMs, making LMs an important candidate
for conductive filler materials for flexible sensors. Furthermore, by mixing highly conductive particles (Cu,
Ag) with LMs, the conductivity of LMs can be enhanced even more. For example, the oxidation film of Cu
particles and eutectic gallium-indium alloy (EGaIn) is removed in the presence of an alkaline solution and
electrical potential, and the conductivity of the mutually doped material is 1.8 times higher than that of pure
[76]
EGaIn . This method also allows the configuration of materials with different Cu contents, offering
enhanced conductivity potential for LMs.
Gallium-based oxide layer
Exposure of LMs to air results in the formation of an amorphous oxide film (Ga O ) on the surface of
3
2
[81]
droplets. Among many structures, β-Ga O is the most thermodynamically stable . On the one hand, the
3
2
oxide film passivates to protect the LM inside while preventing droplet aggregation and facilitating the
formation and stable existence of NPs. On the other hand, Ga O has an intrinsic n-type conductivity , and
[82]
2
3
its conductivity depends mainly on the carrier concentration and electron mobility of the material , both
[83]
of which would change when Ga O is exposed to the target gas, causing changes in Ga O conductivity or
3
2
3
2
resistance, so Ga O can be used in gas detection sensors. The mechanisms by which Ga O detects target
2
3
3
2
gases can be divided into three categories, with different mechanisms dominating at different
[84]
temperatures as follows: (i) At high temperatures, the ambient oxygen concentration and Ga O oxygen
2
3
concentration are in dynamic equilibrium, when the ambient oxygen concentration decreases, the
concentration of positively ionized oxygen defects within Ga O increases and its delocalized electrons
3
2
increase, leading to an increase in the conductivity of the material; (ii) When the temperature drops,
reducing gases, such as CH , will attach to the crystal surface and react with Ga O , which will release
4
2
3
conduction electrons within the material and increase its conductivity; (iii) When the temperature is lower,