Page 30 - Read Online
P. 30
Page 10 of 16 Allam et al. Plast Aesthet Res 2024;11:19 https://dx.doi.org/10.20517/2347-9264.2024.21
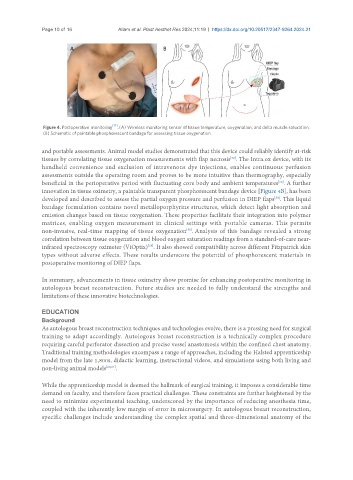
Figure 4. Postoperative monitoring [17] . (A) Wireless monitoring sensor of tissue temperature, oxygenation, and delta muscle saturation;
(B) Schematic of paintable phosphorescent bandage for assessing tissue oxygenation.
and portable assessments. Animal model studies demonstrated that this device could reliably identify at-risk
tissues by correlating tissue oxygenation measurements with flap necrosis . The Intra.ox device, with its
[52]
handheld convenience and exclusion of intravenous dye injections, enables continuous perfusion
assessments outside the operating room and proves to be more intuitive than thermography, especially
[52]
beneficial in the perioperative period with fluctuating core body and ambient temperatures . A further
innovation in tissue oximetry, a paintable transparent phosphorescent bandage device [Figure 4B], has been
developed and described to assess the partial oxygen pressure and perfusion in DIEP flaps . This liquid
[58]
bandage formulation contains novel metalloporphyrins structures, which detect light absorption and
emission changes based on tissue oxygenation. These properties facilitate their integration into polymer
matrices, enabling oxygen measurement in clinical settings with portable cameras. This permits
non-invasive, real-time mapping of tissue oxygenation . Analysis of this bandage revealed a strong
[58]
correlation between tissue oxygenation and blood oxygen saturation readings from a standard-of-care near-
infrared spectroscopy oximeter (ViOptix) . It also showed compatibility across different Fitzpatrick skin
[58]
types without adverse effects. These results underscore the potential of phosphorescent materials in
postoperative monitoring of DIEP flaps.
In summary, advancements in tissue oximetry show promise for enhancing postoperative monitoring in
autologous breast reconstruction. Future studies are needed to fully understand the strengths and
limitations of these innovative biotechnologies.
EDUCATION
Background
As autologous breast reconstruction techniques and technologies evolve, there is a pressing need for surgical
training to adapt accordingly. Autologous breast reconstruction is a technically complex procedure
requiring careful perforator dissection and precise vessel anastomosis within the confined chest anatomy.
Traditional training methodologies encompass a range of approaches, including the Halsted apprenticeship
model from the late 1,800s, didactic learning, instructional videos, and simulations using both living and
non-living animal models [60,61] .
While the apprenticeship model is deemed the hallmark of surgical training, it imposes a considerable time
demand on faculty, and therefore faces practical challenges. These constraints are further heightened by the
need to minimize experimental teaching, underscored by the importance of reducing anesthesia time,
coupled with the inherently low margin of error in microsurgery. In autologous breast reconstruction,
specific challenges include understanding the complex spatial and three-dimensional anatomy of the