Page 144 - Read Online
P. 144
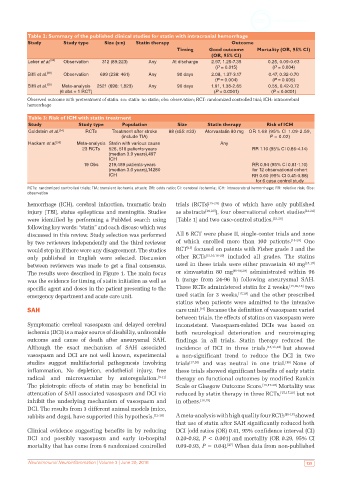
Table 2: Summary of the published clinical studies for statin with intracranial hemorrhage
Study Study type Size (s:n) Statin therapy Outcome
Timing Good outcome Mortality (OR, 95% CI)
(OR, 95% CI)
Leker et al. [33] Observation 312 (89:223) Any At discharge 2.97, 1.25-7.35 0.25, 0.09-0.63
(P = 0.015) (P = 0.004)
Biffi et al. [28] Observation 699 (238: 461) Any 90 days 2.08, 1.37-3.17 0.47, 0.32-0.70
(P = 0.004) (P = 0.005)
Biffi et al. [28] Meta-analysis 2521 (698: 1,823) Any 90 days 1.91, 1.38-2.65 0.55, 0.42-0.72
(6 obs + 1 RCT) (P < 0.0001) (P < 0.0001)
Observed outcome with pretreatment of statin. s:n: statin: no statin; obs: observation; RCT: randomized controlled trial; ICH: intracerebral
hemorrhage
Table 3: Risk of ICH with statin treatment
Study Study type Population Size Statin therapy Risk of ICH
Goldstein et al. [34] RCTs Treatment after stroke 88 (s55: n33) Atorvastatin 80 mg OR 1.68 (95% CI 1.09-2.59,
(include TIA) P = 0.02)
Hackam et al. [35] Meta-analysis Statin with various cause Any
23 RCTs 526, 518 patients-years RR 1.10 (95% CI 0.86-4.14)
(median 3.9 years),497
ICH
19 Obs 219,459 patients-years RR 0.94 (95% CI 0.81-1.10)
(median 3.0 years),14280 for 12 observational cohort
ICH RR 0.60 (95% CI 0.41-0.88)
for 6 case control study
RCTs: randomized controlled trials; TIA: transient ischemia attach; OR: odds ratio; CI: cerebral ischemia; ICH: intracerebral hemorrhage; RR: relative risk; Obs:
observation
hemorrhage (ICH), cerebral infarction, traumatic brain trials (RCTs) [15-20] (two of which have only published
injury (TBI), status epilepticus and meningitis. Studies as abstracts [19,20] ), four observational cohort studies [21-24]
were identified by performing a PubMed search using [Table 1] and two case-control studies. [25,26]
following key words: “statin” and each disease which was
discussed in this review. Study selection was performed All 6 RCT were phase II, single-center trials and none
by two reviewers independently and the third reviewer of which enrolled more than 100 patients. [15-20] One
[17]
would step in if there were any disagreement. The studies RCT focused on patents with Fisher grade 3 and the
only published in English were selected. Discussion other RCTs [15,16,18-20] included all grades. The statins
between reviewers was made to get a final consensus. used in these trials were either pravastain 40 mg [15,19]
The results were described in Figure 1. The main focus or simvastatin 80 mg [16-18,20] administrated within 96
was the evidence for timing of statin initiation as well as h (range from 24-96 h) following aneurysmal SAH.
specific agent and doses in the patient presenting to the Three RCTs administered statin for 2 weeks, [15,16,18] two
emergency department and acute care unit. used statin for 3 weeks, [17,20] and the other prescribed
statins when patients were admitted to the intensive
SAH care unit. Because the definition of vasospasm varied
[19]
between trials, the effects of statins on vasospasm were
Symptomatic cerebral vasospasm and delayed cerebral inconsistent. Vasospasm-related DCIs was based on
ischemia (DCI) is a major source of disability, unfavorable both neurological deterioration and neuroimaging
outcome and cause of death after aneurysmal SAH. findings in all trials. Statin therapy reduced the
Although the exact mechanism of SAH associated incidence of DCI in three trials, [15,16,18] but showed
vasospasm and DCI are not well known, experimental a non-significant trend to reduce the DCI in two
studies suggest multifactorial pathogenesis involving trials [17,20] and was neutral in one trial. [18] None of
inflammation, No depletion, endothelial injury, free these trials showed significant benefits of early statin
radical and microvascular by autoregulation. [9-11] therapy on functional outcomes by modified Rankin
The pleiotropic effects of statin may be beneficial in Scale or Glasgow Outcome Score. [15,17-20] Mortality was
attenuation of SAH associated vasospasm and DCI via reduced by statin therapy in three RCTs, [15,17,20] but not
inhibit the underlying mechanism of vasospasm and in others. [18,19]
DCI. The results from 3 different animal models (mice,
rabbits and dogs), have supported this hypothesis. [12-14] A meta-analysis with high quality four RCTs [15-18] showed
that use of statin after SAH significantly reduced both
Clinical evidence suggesting benefits in by reducing DCI [odd ratios (OR) 0.41, 95% confidence interval (CI)
DCI and possibly vasospasm and early in-hospital 0.20-0.82, P < 0.001] and mortality (OR 0.29, 95% CI
[27]
mortality that has come from 6 randomized controlled 0.09-0.93, P = 0.04). When data from non-published
Neuroimmunol Neuroinflammation | Volume 3 | June 20, 2016 135