Page 162 - Read Online
P. 162
a b
a b
c d
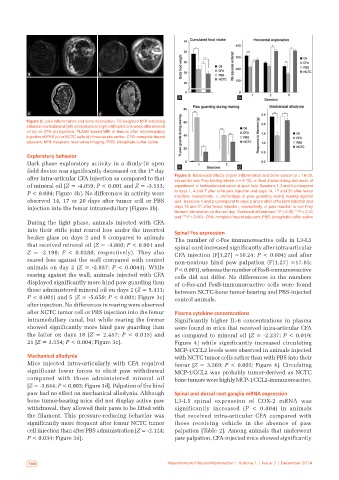
Figure 2: Joint inflammation and bone destruction. T2-weighted MRI indicating
edema in contralateral (left) and ipsilateral (right) stifle joint one week after mineral
oil (a) or CFA (b) injection. FLASH‑based MRI of femurs after intramedullary
injection of PBS (c) or NCTC cells (d) three weeks earlier. CFA: complete freund
adjuvant; MRI: magnetic resonance imaging; PBS: phosphate buffer saline
Exploratory behavior
Dark phase exploratory activity in a dimly‑lit open c
st
field device was significantly decreased on the 1 day d
after intra‑articular CFA injection as compared to that Figure 3: Behavioral effects of joint inflammation and bone cancer (n = 18‑23,
except for von Frey testing where n = 9‑13). a: food intake during last week of
of mineral oil [Z = ‑4.059; P < 0.001 and Z = ‑3.553; experiment. b: horizontal exploration of open field. Sessions 1, 2 and 3 correspond
P < 0.004; Figure 3b]. No differences in activity were to days 1, 4 and 7 after stifle joint injection and days 14, 17 and 20 after femur
injection, respectively. c: percentage of paw guarding during rearing against
observed 14, 17 or 20 days after tumor cell or PBS wall. Sessions 1 and 2 correspond to days 2 and 5 after stifle joint injection and
injection into the femur intramedullary [Figure 3b]. days 18 and 21 after femur injection, respectively. d: paw reaction to von Frey
filament stimulation on the last day. Statistical differences: *P < 0.05, **P < 0.01
and ***P < 0.001. CFA: complete freund adjuvant; PBS: phosphate buffer saline
During the light phase, animals injected with CFA
into their stifle joint reared less under the inverted Spinal Fos expression
beaker glass on days 2 and 5 compared to animals The number of c‑Fos immunoreactive cells in L3‑L5
that received mineral oil (Z = ‑4.860; P < 0.001 and spinal cord increased significantly after intra‑articular
Z = ‑2.198; P < 0.0280, respectively). They also CFA injection (F[1,27] =10.24; P < 0.004) and after
reared less against the wall compared with control non‑noxious hind paw palpation (F[1,27] =17.85;
animals on day 2 (Z = ‑2.857; P < 0.0043). While P < 0.001), whereas the number of FosB‑immunoreactive
rearing against the wall, animals injected with CFA cells did not differ. No differences in the numbers
displayed significantly more hind paw guarding than of c‑Fos‑and FosB‑immunoreactive cells were found
those administered mineral oil on days 2 (Z = 5.411; between NCTC‑bone tumor‑bearing and PBS‑injected
P < 0.001) and 5 [Z = ‑5.650; P < 0.001; Figure 3c] control animals.
after injection. No differences in rearing were observed
after NCTC tumor cell or PBS injection into the femur Plasma cytokine concentrations
intramedullary canal, but while rearing the former Significantly higher IL‑6 concentrations in plasma
showed significantly more hind paw guarding than were found in mice that received intra‑articular CFA
the latter on days 18 (Z = 2.457; P < 0.015) and as compared to mineral oil [Z = -2.237; P < 0.019;
21 [Z = 3.554; P < 0.004; Figure 3c]. Figure 4] while significantly increased circulating
MCP‑1/CCL2 levels were observed in animals injected
Mechanical allodynia with NCTC tumor cells rather than with PBS into their
Mice injected intra‑articularly with CFA required femur [Z = 3.269; P < 0.002; Figure 4]. Circulating
significant lower forces to elicit paw withdrawal MCP‑1/CCL2 was probably tumor‑derived as NCTC
compared with those administered mineral oil bone tumors were highly MCP‑1/CCL2‑immunoreactive.
[Z = ‑3.644; P < 0.003; Figure 3d]. Palpation of the hind
paw had no effect on mechanical allodynia. Although Spinal and dorsal root ganglia mRNA expression
bone tumor‑bearing mice did not display active paw L3‑L5 spinal expression of COX‑2 mRNA was
withdrawal, they allowed their paws to be lifted with significantly increased (P < 0.004) in animals
the filament. This pressure‑reducing behavior was that received intra‑articular CFA compared with
significantly more frequent after femur NCTC tumor those receiving vehicle in the absence of paw
cell injection than after PBS administration [Z = ‑2.124; palpation [Table 2]. Among animals that underwent
P < 0.034; Figure 3d]. paw palpation, CFA‑injected mice showed significantly
156 Neuroimmunol Neuroinflammation | Volume 1 | Issue 3 | December 2014