Page 714 - Read Online
P. 714
Masiero et al. Mini-invasive Surg 2020;4:71 I http://dx.doi.org/10.20517/2574-1225.2020.56 Page 7 of 12
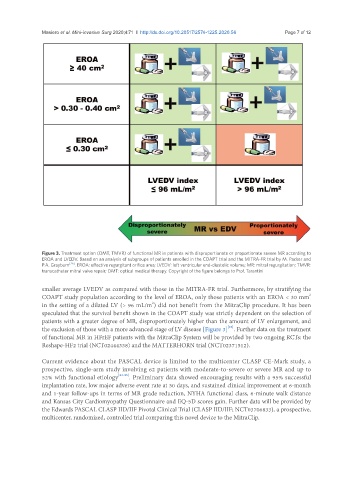
Figure 3. Treatment option (OMT, TMVR) of functional MR in patients with disproportionate or proportionate severe MR according to
EROA and LVEDV. Based on an analysis of subgroups of patients enrolled in the COAPT trial and the MITRA-FR trial by M. Packer and
P.A. Grayburn [26] . EROA: effective regurgitant orifice area; LVEDV: left ventricular end-diastolic volume; MR: mitral regurgitation; TMVR:
transcatheter mitral valve repair; OMT: optical medical therapy. Copyright of the figure belongs to Prof. Tarantini
smaller average LVEDV as compared with those in the MITRA-FR trial. Furthermore, by stratifying the
2
COAPT study population according to the level of EROA, only those patients with an EROA < 30 mm
2
in the setting of a dilated LV (> 96 mL/m ) did not benefit from the MitraClip procedure. It has been
speculated that the survival benefit shown in the COAPT study was strictly dependent on the selection of
patients with a greater degree of MR, disproportionately higher than the amount of LV enlargement, and
[22]
the exclusion of those with a more advanced stage of LV disease [Figure 3] . Further data on the treatment
of functional MR in HFrEF patients with the MitraClip System will be provided by two ongoing RCTs: the
Reshape-HF2 trial (NCT02444338) and the MATTERHORN trial (NCT02371512).
Current evidence about the PASCAL device is limited to the multicenter CLASP CE-Mark study, a
prospective, single-arm study involving 62 patients with moderate-to-severe or severe MR and up to
52% with functional etiology [23-25] . Preliminary data showed encouraging results with a 95% successful
implantation rate, low major adverse event rate at 30 days, and sustained clinical improvement at 6-month
and 1-year follow-ups in terms of MR grade reduction, NYHA functional class, 6-minute walk distance
and Kansas City Cardiomyopathy Questionnaire and EQ-5D scores gain. Further data will be provided by
the Edwards PASCAL CLASP IID/IIF Pivotal Clinical Trial (CLASP IID/IIF; NCT03706833), a prospective,
multicenter, randomized, controlled trial comparing this novel device to the MitraClip.