Page 711 - Read Online
P. 711
Page 4 of 12 Masiero et al. Mini-invasive Surg 2020;4:71 I http://dx.doi.org/10.20517/2574-1225.2020.56
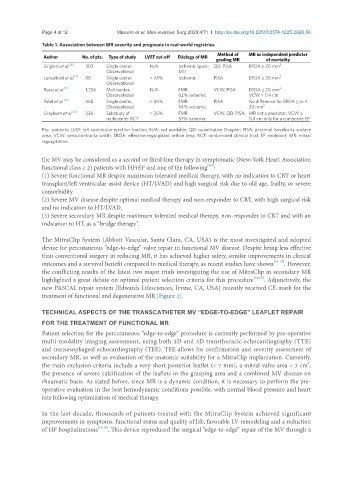
Table 1. Association between MR severity and prognosis in real-world registries
Method of MR as independent predictor
Author No. of pts. Type of study LVEF cut-off Etiology of MR
grading MR of mortality
Grigioni et al. [6] 303 Single center, N/A Ischemic (post- QD, PISA EROA ≥ 20 mm 2
Observational MI)
Lancellotti et al. [7] 98 Single center, < 45% Ischemic PISA EROA ≥ 20 mm 2
Observational
Rossi et al. [5] 1,256 Multicenter, N/A FMR VCW, PISA EROA ≥ 20 mm 2
Observational 62% ischemic VCW > 0.4 cm
Patel et al. [10] 558 Single center, < 35% FMR PISA No difference for EROA ≥ or <
Observational 54% ischemic 20 mm 2
Grayburn et al. [11] 336 Substudy of < 35% FMR VCW, QD, PISA MR not a predictor; VCW ≥
multicenter RCT 57% ischemic 0.4 cm only for a composite EP
Pts.: patients; LVEF: left ventricular ejection fraction; N/A: not available; QD: quantitative Doppler; PISA: proximal isovelocity surface
area; VCW: vena contracta width; EROA: effective regurgitant orifice area; RCT: randomized clinical trial; EP: endpoint; MR: mitral
regurgitation
the MV may be considered as a second or third-line therapy in symptomatic (New-York Heart Association
functional class ≥ 2) patients with HFrEF and one of the following :
[1,9]
(1) Severe functional MR despite maximum tolerated medical therapy, with no indication to CRT or heart
transplant/left ventricular assist device (HT/LVAD) and high surgical risk due to old age, frailty, or severe
comorbidity.
(2) Severe MV disease despite optimal medical therapy and non-responder to CRT, with high surgical risk
and no indication to HT/LVAD.
(3) Severe secondary MR despite maximum tolerated medical therapy, non-responder to CRT and with an
indication to HT, as a “bridge therapy”.
The MitraClip System (Abbott Vascular, Santa Clara, CA, USA) is the most investigated and adopted
device for percutaneous “edge-to-edge” valve repair in functional MV disease. Despite being less effective
than conventional surgery in reducing MR, it has achieved higher safety, similar improvements in clinical
outcomes and a survival benefit compared to medical therapy, as recent studies have shown [13-15] . However,
the conflicting results of the latest two major trials investigating the use of MitraClip in secondary MR
highlighted a great debate on optimal patient selection criteria for this procedure [14,15] . Adjunctively, the
new PASCAL repair system (Edwards Lifesciences, Irvine, CA, USA) recently received CE-mark for the
treatment of functional and degenerative MR [Figure 2].
TECHNICAL ASPECTS OF THE TRANSCATHETER MV “EDGE-TO-EDGE” LEAFLET REPAIR
FOR THE TREATMENT OF FUNCTIONAL MR
Patient selection for the percutaneous “edge-to-edge” procedure is currently performed by pre-operative
multi-modality imaging assessment, using both 2D and 3D transthoracic echocardiography (TTE)
and transesophageal echocardiography (TEE). TEE allows for confirmation and severity assessment of
secondary MR, as well as evaluation of the anatomic suitability for a MitraClip implantation. Currently,
the main exclusion criteria include a very short posterior leaflet (< 7 mm), a mitral valve area < 3 cm ,
2
the presence of severe calcification of the leaflets in the grasping area and a combined MV disease on
rheumatic basis. As stated before, since MR is a dynamic condition, it is necessary to perform the pre-
operative evaluation in the best hemodynamic conditions possible, with normal blood pressure and heart
rate following optimization of medical therapy.
In the last decade, thousands of patients treated with the MitraClip System achieved significant
improvements in symptoms, functional status and quality of life, favorable LV remodeling and a reduction
of HF hospitalizations [13,16] . This device reproduced the surgical “edge-to-edge” repair of the MV through a