Page 62 - Read Online
P. 62
Riachi et al. Mini-invasive Surg 2023;7:14 https://dx.doi.org/10.20517/2574-1225.2022.120 Page 7 of 11
(P < 0.01) 0.652) 0.659) (P = 1.00) 0.014)
0.040)
[71]
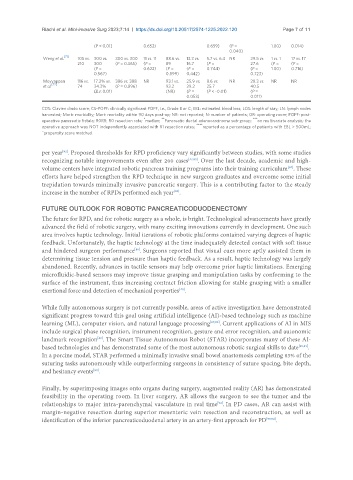
Weng et al. 105 vs. 300 vs. 300 vs. 300 11 vs. 11 88.6 vs. 13.3 vs. 5.7 vs. 6.4 NR 29.5 vs. 1 vs. 1 17 vs. 17
210 300 (P = 0.365) (P = 89 16.7 (P = 27.6 (P = (P =
(P = 0.622) (P = (P = 0.744) (P = 1.00) 0.716)
0.567) 0.899) 0.442) 0.723)
Meyyappan 116 vs. 17.2% vs. 386 vs. 388 NR 93.1 vs. 25.9 vs. 8.6 vs. NR 28.3 vs. NR NR
et al. [72] 74 34.3% (P = 0.896) 93.2 39.2 25.7 40.5
(P < 0.01) (NR) (P = (P < -0.01) (P =
****
0.053) 0.011)
CDS: Clavien dindo score; CS-POPF: clinically significant POPF, i.e., Grade B or C; EBL: estimated blood loss; LOS: length of stay; LN: lymph nodes
harvested; Morb: morbidity; Mort: mortality within 90 days post-op; NR: not reported; N: number of patients; OR: operating room; POPF: post-
*
**
operative pancreatic fistula; R0RR: R0 resection rate; median; Pancreatic ductal adenocarcinoma sub-group; *** on multivariate analysis; the
operative approach was NOT independently associated with R1 resection rates; **** reported as a percentage of patients with EBL > 500mL;
^propensity score matched.
per year . Proposed thresholds for RPD proficiency vary significantly between studies, with some studies
[82]
recognizing notable improvements even after 200 cases [47,83] . Over the last decade, academic and high-
volume centers have integrated robotic pancreas training programs into their training curriculum . These
[84]
efforts have helped strengthen the RPD technique in new surgeon graduates and overcome some initial
trepidation towards minimally invasive pancreatic surgery. This is a contributing factor to the steady
increase in the number of RPDs performed each year .
[85]
FUTURE OUTLOOK FOR ROBOTIC PANCREATICODUODENECTOMY
The future for RPD, and for robotic surgery as a whole, is bright. Technological advancements have greatly
advanced the field of robotic surgery, with many exciting innovations currently in development. One such
area involves haptic technology. Initial iterations of robotic platforms contained varying degrees of haptic
feedback. Unfortunately, the haptic technology at the time inadequately detected contact with soft tissue
and hindered surgeon performance . Surgeons reported that visual cues more aptly assisted them in
[86]
determining tissue tension and pressure than haptic feedback. As a result, haptic technology was largely
abandoned. Recently, advances in tactile sensors may help overcome prior haptic limitations. Emerging
microfluidic-based sensors may improve tissue grasping and manipulation tasks by conforming to the
surface of the instrument, thus increasing contract friction allowing for stable grasping with a smaller
exertional force and detection of mechanical properties .
[40]
While fully autonomous surgery is not currently possible, areas of active investigation have demonstrated
significant progress toward this goal using artificial intelligence (AI)-based technology such as machine
learning (ML), computer vision, and natural language processing [87,88] . Current applications of AI in MIS
include surgical phase recognition, instrument recognition, gesture and error recognition, and autonomic
[89]
landmark recognition . The Smart Tissue Autonomous Robot (STAR) incorporates many of these AI-
based technologies and has demonstrated some of the most autonomous robotic surgical skills to date [90,91] .
In a porcine model, STAR performed a minimally invasive small bowel anastomosis completing 83% of the
suturing tasks autonomously while outperforming surgeons in consistency of suture spacing, bite depth,
and hesitancy events .
[90]
Finally, by superimposing images onto organs during surgery, augmented reality (AR) has demonstrated
feasibility in the operating room. In liver surgery, AR allows the surgeon to see the tumor and the
[92]
relationships to major intra-parenchymal vasculature in real time . In PD cases, AR can assist with
margin-negative resection during superior mesenteric vein resection and reconstruction, as well as
identification of the inferior pancreaticoduodenal artery in an artery-first approach for PD [93,94] .