Page 81 - Read Online
P. 81
Nguyen et al. J Transl Genet Genom 2018;2:19 I http://dx.doi.org/10.20517/jtgg.2018.20 Page 7 of 11
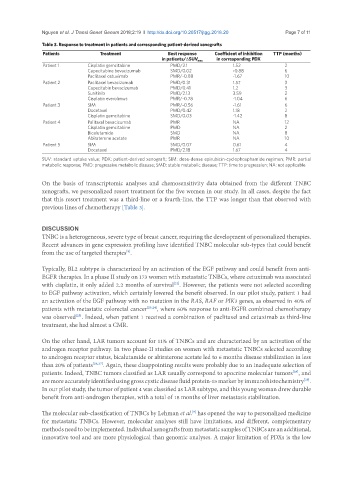
Table 3. Response to treatment in patients and corresponding patient-derived xenografts
Patients Treatment Best response Coefficient of inhibition TTP (months)
in corresponding PDX
in patients/∆SUV max
Patient 1 Cisplatin gemcitabine PMD/2.1 1.52 2
Capecitabine bevacizumab SMD/0.02 -0.88 6
Paclitaxel cetuximab PMR/-0.88 -1.67 10
Patient 2 Paclitaxel bevacizumab PMD/0.31 1.57 3
Capecitabin bevacizumab PMD/0.41 1.2 3
Sunitinib PMD/2.13 3.59 2
Cisplatin everolimus PMR/-0.78 -1.04 6
Patient 3 SIM PMR/-0.56 -1.61 6
Docetaxel PMD/0.42 1.18 2
Cisplatin gemcitabine SMD/0.03 -1.42 8
Patient 4 Palitaxel bevacizumab PMR NA 12
Cisplatin gemcitabine PMD NA 2
Bicalutamide SMD NA 8
Abiraterone acetate PMR NA 10
Patient 5 SIM SMD/0.07 0.61 4
Docetaxel PMD/2.18 1.67 4
SUV: standard uptake value; PDX: patient-derived xenograft; SIM: dose-dense epirubicin-cyclophosphamide regimen; PMR: partial
metabolic response; PMD: progressive metabolic disease; SMD: stable metabolic disease; TTP: time to progression; NA: not applicable
On the basis of transcriptomic analyses and chemosensitivity data obtained from the different TNBC
xenografts, we personalized resort treatment for the five women in our study. In all cases, despite the fact
that this resort treatment was a third-line or a fourth-line, the TTP was longer than that observed with
previous lines of chemotherapy [Table 3].
DISCUSSION
TNBC is a heterogeneous, severe type of breast cancer, requiring the development of personalized therapies.
Recent advances in gene expression profiling have identified TNBC molecular sub-types that could benefit
from the use of targeted therapies .
[9]
Typically, BL2 subtype is characterized by an activation of the EGF pathway and could benefit from anti-
EGFR therapies. In a phase II study on 173 women with metastatic TNBCs, where cetuximab was associated
with cisplatin, it only added 2.2 months of survival . However, the patients were not selected according
[22]
to EGF pathway activation, which certainly lowered the benefit observed. In our pilot study, patient 1 had
an activation of the EGF pathway with no mutation in the RAS, RAF or PIK3 genes, as observed in 40% of
patients with metastatic colorectal cancer [23,24] , where 60% response to anti-EGFR combined chemotherapy
was observed . Indeed, when patient 1 received a combination of paclitaxel and cetuximab as third-line
[25]
treatment, she had almost a CMR.
On the other hand, LAR tumors account for 11% of TNBCs and are characterized by an activation of the
androgen receptor pathway. In two phase-II studies on women with metastatic TNBCs selected according
to androgen receptor status, bicalutamide or abiraterone acetate led to 6 months disease stabilization in less
than 20% of patients [26,27] . Again, these disappointing results were probably due to an inadequate selection of
patients. Indeed, TNBC tumors classified as LAR usually correspond to apocrine molecular tumors , and
[28]
are more accurately identified using gross cystic disease fluid protein-15 marker by immunohistochemistry .
[29]
In our pilot study, the tumor of patient 4 was classified as LAR subtype, and this young woman drew durable
benefit from anti-androgen therapies, with a total of 18 months of liver metastasis stabilization.
The molecular sub-classification of TNBCs by Lehman et al. has opened the way to personalized medicine
[9]
for metastatic TNBCs. However, molecular analyses still have limitations, and different, complementary
methods need to be implemented. Individual xenografts from metastatic samples of TNBCs are an additional,
innovative tool and are more physiological than genomic analyses. A major limitation of PDXs is the low