Page 28 - Read Online
P. 28
Page 6 of 15 Wang et al. J Mater Inf 2023;3:3 https://dx.doi.org/10.20517/jmi.2022.45
Figure 1. Computational results of the stability of LSCF-6428 under 10 ppm SO in terms of temperature in a) dry air and b) argon.
2
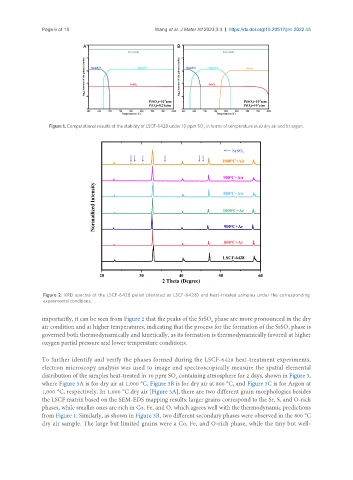
Figure 2. XRD spectra of the LSCF-6428 pellet (denoted as LSCF-6428) and heat-treated samples under the corresponding
experimental conditions.
importantly, it can be seen from Figure 2 that the peaks of the SrSO phase are more pronounced in the dry
4
air condition and at higher temperatures, indicating that the process for the formation of the SrSO phase is
4
governed both thermodynamically and kinetically, as its formation is thermodynamically favored at higher
oxygen partial pressure and lower temperature conditions.
To further identify and verify the phases formed during the LSCF-6428 heat-treatment experiments,
electron microscopy analysis was used to image and spectroscopically measure the spatial elemental
distribution of the samples heat-treated in 10 ppm SO containing atmosphere for 2 days, shown in Figure 3,
2
where Figure 3A is for dry air at 1,000 °C, Figure 3B is for dry air at 800 °C, and Figure 3C is for Argon at
1,000 °C, respectively. In 1,000 °C dry air [Figure 3A], there are two different grain morphologies besides
the LSCF matrix based on the SEM-EDS mapping results: larger grains correspond to the Sr, S, and O-rich
phases, while smaller ones are rich in Co, Fe, and O, which agrees well with the thermodynamic predictions
from Figure 1. Similarly, as shown in Figure 3B, two different secondary phases were observed in the 800 °C
dry air sample. The large but limited grains were a Co, Fe, and O-rich phase, while the tiny but well-