Page 135 - Read Online
P. 135
Page 4 of 18 Chong et al. J Mater Inf 2023;3:21 https://dx.doi.org/10.20517/jmi.2023.17
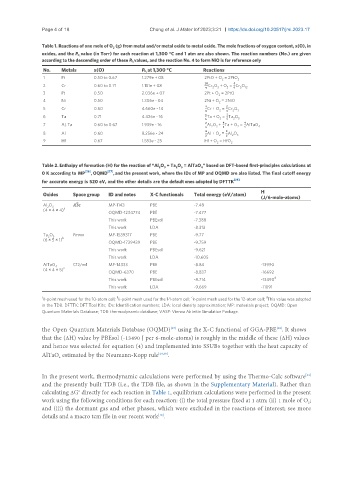
Table 1. Reactions of one mole of O (g) from metal and/or metal oxide to metal oxide. The mole fractions of oxygen content, x(O), in
2
oxides, and the P value (in Torr) for each reaction at 1,300 °C and 1 atm are also shown. The reaction numbers (No.) are given
O 2
according to the descending order of these P values, and the reaction No. 4 to form NiO is for reference only
O
O 2
2
No. Metals x(O) P at 1,300 °C Reactions
O 2
1 Pt 0.50 to 0.67 1.279e + 08 2PtO + O = 2PtO 2
2
2 Cr 0.60 to 0.71 1.151e + 08 9Cr O + O = 9Cr O 12
5
2
3
2
3 Pt 0.50 2.036e + 07 2Pt + O = 2PtO
2
4 Ni 0.50 1.306e - 04 2Ni + O = 2NiO
2
5 Cr 0.60 4.660e - 14 9Cr + O = 9Cr O
2 2 3
6 Ta 0.71 4.426e - 16 9Ta + O = 9Ta O 5
2
2
7 Al, Ta 0.60 to 0.67 1.939e - 16 9Al O + 9Ta + O = 9AlTaO
2 3 2 4
8 Al 0.60 8.256e - 24 9Al + O = 9Al O 3
2
2
9 Hf 0.67 1.553e - 25 Hf + O = HfO
2 2
Table 2. Enthalpy of formation (H) for the reaction of “Al O + Ta O = AlTaO ” based on DFT-based first-principles calculations at
3
2
5
2
4
0 K according to MP [26] , OQMD [27] , and the present work, where the IDs of MP and OQMD are also listed. The final cutoff energy
for accurate energy is 520 eV, and the other details are the default ones adopted by DFTTK [25]
H
Oxides Space group ID and notes X-C functionals Total energy (eV/atom)
(J/6-mole-atoms)
Al O 3 R3C MP-1143 PBE -7.48
2
(4 × 4 × 4) a
OQMD-1234774 PBE -7.477
This work PBEsol -7.388
This work LDA -8.313
Ta O 5 Pmmn MP-1539317 PBE -9.77
2
(6 × 5 × 1) b
OQMD-1739439 PBE -9.759
This work PBEsol -9.621
This work LDA -10.605
AlTaO C12/m1 MP-14333 PBE -8.84 -13990
4
(4 × 4 × 5) c
OQMD-6370 PBE -8.837 -16692
d
This work PBEsol -8.714 -13490
This work LDA -9.669 -11091
a b c d
k-point mesh used for the 10-atom cell; k-point mesh used for the 14-atom cell; k-point mesh used for the 12-atom cell; This value was adopted
in the TDB. DFTTK: DFT Tool Kits; IDs: Identification numbers; LDA: local density approximation; MP: materials project; OQMD: Open
Quantum Materials Database; TDB: thermodynamic database; VASP: Vienna Ab initio Simulation Package.
the Open Quantum Materials Database (OQMD) using the X-C functional of GGA-PBE . It shows
[27]
[28]
that the (ΔH) value by PBEsol (-13490 J per 6-mole-atoms) is roughly in the middle of these (ΔH) values
and hence was selected for equation (4) and implemented into SSUB5 together with the heat capacity of
AlTaO estimated by the Neumann-Kopp rule [29,30] .
4
In the present work, thermodynamic calculations were performed by using the Thermo-Calc software [31]
and the presently built TDB (i.e., the TDB file, as shown in the Supplementary Material). Rather than
calculating ΔG directly for each reaction in Table 1, equilibrium calculations were performed in the present
o
work using the following conditions for each reaction: (i) the total pressure fixed at 1 atm; (ii) 1 mole of O ;
2
and (iii) the dormant gas and other phases, which were excluded in the reactions of interest; see more
details and a macro tcm file in our recent work .
[18]