Page 136 - Read Online
P. 136
Chong et al. J Mater Inf 2023;3:21 https://dx.doi.org/10.20517/jmi.2023.17 Page 5 of 18
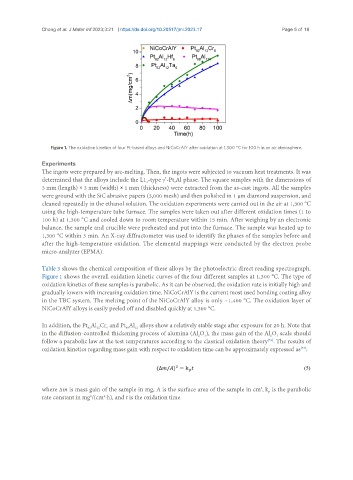
Figure 1. The oxidation kinetics of four Pt-based alloys and NiCoCrAlY after oxidation at 1,300 °C for 100 h in an air atmosphere.
Experiments
The ingots were prepared by arc-melting. Then, the ingots were subjected to vacuum heat treatments. It was
determined that the alloys include the L1 -type γ’-Pt Al phase. The square samples with the dimensions of
3
2
3 mm (length) × 3 mm (width) × 1 mm (thickness) were extracted from the as-cast ingots. All the samples
were ground with the SiC abrasive papers (3,000 mesh) and then polished in 1 μm diamond suspension, and
cleaned repeatedly in the ethanol solution. The oxidation experiments were carried out in the air at 1,300 °C
using the high-temperature tube furnace. The samples were taken out after different oxidation times (1 to
100 h) at 1,300 °C and cooled down to room temperature within 15 min. After weighing by an electronic
balance, the sample and crucible were preheated and put into the furnace. The sample was heated up to
1,300 °C within 3 min. An X-ray diffractometer was used to identify the phases of the samples before and
after the high-temperature oxidation. The elemental mappings were conducted by the electron probe
micro-analyzer (EPMA).
Table 3 shows the chemical composition of these alloys by the photoelectric direct reading spectrograph.
Figure 1 shows the overall oxidation kinetic curves of the four different samples at 1,300 °C. The type of
oxidation kinetics of these samples is parabolic. As it can be observed, the oxidation rate is initially high and
gradually lowers with increasing oxidation time. NiCoCrAlY is the current most used bonding coating alloy
in the TBC system. The melting point of the NiCoCrAlY alloy is only ~1,400 °C. The oxidation layer of
NiCoCrAlY alloys is easily peeled off and disabled quickly at 1,300 °C.
In addition, the Pt Al Cr and Pt Al alloys show a relatively stable stage after exposure for 20 h. Note that
12
12
88
6
82
in the diffusion-controlled thickening process of alumina (Al O ), the mass gain of the Al O scale should
2
3
2
3
[32]
follow a parabolic law at the test temperatures according to the classical oxidation theory . The results of
oxidation kinetics regarding mass gain with respect to oxidation time can be approximately expressed as :
[33]
where Δm is mass gain of the sample in mg, A is the surface area of the sample in cm , k is the parabolic
2
p
rate constant in mg /(cm ·h), and t is the oxidation time.
4
2