Page 1006 - Read Online
P. 1006
Agbo et al. J Cancer Metastasis Treat 2019;5:x I http://dx.doi.org/10.20517/2394-4722.2019.35 Page 7 of 11
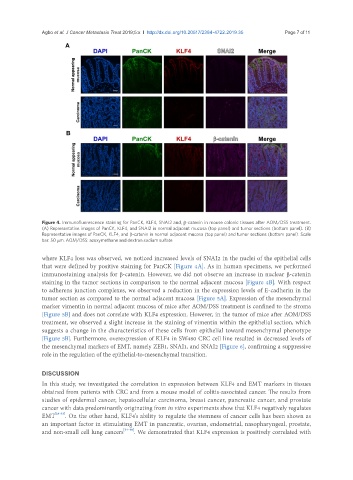
Figure 4. Immunofluorescence staining for PanCK, KLF4, SNAI2 and, β-catenin in mouse colonic tissues after AOM/DSS treatment.
(A) Representative images of PanCK, KLF4, and SNAI2 in normal adjacent mucosa (top panel) and tumor sections (bottom panel). (B)
Representative images of PanCK, KLF4, and β-catenin in normal adjacent mucosa (top panel) and tumor sections (bottom panel). Scale
bar: 50 μm. AOM/DSS: azoxymethane and dextran sodium sulfate
where KLF4 loss was observed, we noticed increased levels of SNAI2 in the nuclei of the epithelial cells
that were defined by positive staining for PanCK [Figure 4A]. As in human specimens, we performed
immunostaining analysis for β-catenin. However, we did not observe an increase in nuclear β-catenin
staining in the tumor sections in comparison to the normal adjacent mucosa [Figure 4B]. With respect
to adherens junction complexes, we observed a reduction in the expression levels of E-cadherin in the
tumor section as compared to the normal adjacent mucosa [Figure 5A]. Expression of the mesenchymal
marker vimentin in normal adjacent mucosa of mice after AOM/DSS treatment is confined to the stroma
[Figure 5B] and does not correlate with KLF4 expression. However, in the tumor of mice after AOM/DSS
treatment, we observed a slight increase in the staining of vimentin within the epithelial section, which
suggests a change in the characteristics of these cells from epithelial toward mesenchymal phenotype
[Figure 5B]. Furthermore, overexpression of KLF4 in SW480 CRC cell line resulted in decreased levels of
the mesenchymal markers of EMT, namely ZEB1, SNAI1, and SNAI2 [Figure 6], confirming a suppressive
role in the regulation of the epithelial-to-mesenchymal transition.
DISCUSSION
In this study, we investigated the correlation in expression between KLF4 and EMT markers in tissues
obtained from patients with CRC and from a mouse model of colitis-associated cancer. The results from
studies of epidermal cancer, hepatocellular carcinoma, breast cancer, pancreatic cancer, and prostate
cancer with data predominantly originating from in vitro experiments show that KLF4 negatively regulates
EMT [28-33] . On the other hand, KLF4’s ability to regulate the stemness of cancer cells has been shown as
an important factor in stimulating EMT in pancreatic, ovarian, endometrial, nasopharyngeal, prostate,
and non-small cell lung cancers [34-40] . We demonstrated that KLF4 expression is positively correlated with