Page 1002 - Read Online
P. 1002
Agbo et al. J Cancer Metastasis Treat 2019;5:x I http://dx.doi.org/10.20517/2394-4722.2019.35 Page 3 of 11
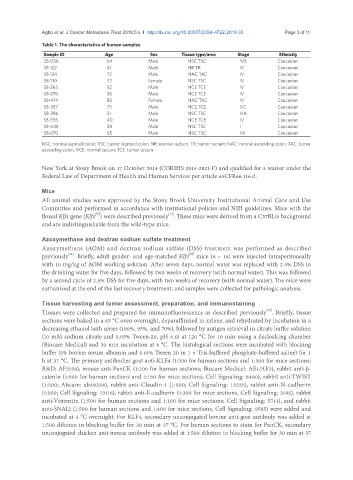
Table 1. The characteristics of human samples
Sample ID Age Sex Tissue type/area Stage Ethnicity
SB-036 64 Male NSC TSC IVB Caucasian
SB-122 41 Male NR TR IV Caucasian
SB-124 72 Male NAC TAC IV Caucasian
SB-130 72 Female NSC TSC IV Caucasian
SB-263 52 Male NCE TCE IV Caucasian
SB-378 36 Male NCE TCE IV Caucasian
SB-474 85 Female NAC TAC IV Caucasian
SB-337 75 Male NCE TCE IIIC Caucasian
SB-396 51 Male NSC TSC IIIA Caucasian
SB-555 40 Male NCE TCE IV Caucasian
SB-645 38 Male NSC TSC I Caucasian
SB-670 65 Male NSC TSC IIA Caucasian
NSC: normal sigmoid colon; TSC: tumor sigmoid colon; NR: normal rectum; TR: tumor rectum; NAC: normal ascending colon; TAC: tumor
ascending colon; NCE: normal cecum; TCE: tumor cecum
New York at Stony Brook on 17 October 2014 (CORIHS 2014-2821-F) and qualified for a waiver under the
Federal Law of Department of Health and Human Services per article 45CFR46.116.d.
Mice
All animal studies were approved by the Stony Brook University Institutional Animal Care and Use
Committee and performed in accordance with institutional policies and NIH guidelines. Mice with the
fl/fl
[12]
floxed Klf4 gene (Klf4 ) were described previously . These mice were derived from a C57BL/6 background
and are indistinguishable from the wild-type mice.
Azoxymethane and dextran sodium sulfate treatment
Azoxymethane (AOM) and dextran sodium sulfate (DSS) treatment was performed as described
[18]
fl/fl
previously . Briefly, adult gender- and age-matched Klf4 mice (n = 16) were injected intraperitoneally
with 10 mg/kg of AOM working solution. After seven days, normal water was replaced with 2.5% DSS in
the drinking water for five days, followed by two weeks of recovery (with normal water). This was followed
by a second cycle of 2.5% DSS for five days, with two weeks of recovery (with normal water). The mice were
euthanized at the end of the last recovery treatment, and samples were collected for pathologic analysis.
Tissue harvesting and tumor assessment, preparation, and immunostaining
Tissues were collected and prepared for immunofluorescence as described previously . Briefly, tissue
[18]
sections were baked in a 65 °C oven overnight, deparaffinized in xylene, and rehydrated by incubation in a
decreasing ethanol bath series (100%, 95%, and 70%), followed by antigen retrieval in citrate buffer solution
(10 mM sodium citrate and 0.05% Tween-20, pH 6.0) at 120 °C for 10 min using a decloaking chamber
(Biocare Medical) and 30 min incubation at 4 °C. The histological sections were incubated with blocking
buffer (5% bovine serum albumin and 0.01% Tween 20 in 1 × Tris-buffered phosphate-buffered saline) for 1
h at 37 °C. The primary antibodies goat anti-KLF4 (1:200 for human sections and 1:300 for mice sections;
R&D: AF3158), mouse anti-PanCK (1:200 for human sections; Biocare Medical: AE1/AE3), rabbit anti-β-
catenin (1:500 for human sections and 1:150 for mice sections; Cell Signaling: 8480), rabbit anti-TWIST
(1:500; Abcam: ab49254), rabbit anti-Claudin-1 (1:500; Cell Signaling: 13255), rabbit anti-N-cadherin
(1:500; Cell Signaling: 13116), rabbit anti-E-cadherin (1:300 for mice sections, Cell Signaling: 3195), rabbit
anti-Vimentin (1:500 for human sections and 1:100 for mice sections; Cell Signaling: 5741), and rabbit
anti-SNAI2 (1:500 for human sections and 1:400 for mice sections, Cell Signaling: 9585) were added and
incubated at 4 °C overnight. For KLF4, secondary unconjugated bovine anti-goat antibody was added at
1:500 dilution in blocking buffer for 30 min at 37 °C. For human sections to stain for PanCK, secondary
unconjugated chicken anti-mouse antibody was added at 1:500 dilution in blocking buffer for 30 min at 37