Page 56 - Read Online
P. 56
Wang et al. Genotoxic NF-kB activation in cancer
at www.nf-kb.org), which participate in a wide range alternative pathway of NF-kB activation relies on the
of physiological and pathological processes, such IKKα homodimer activation in a manner dependent
as cell proliferation, innate and adaptive immune on NF-kB inducing kinase (NIK). Activated IKKα then
responses, inflammation, cell migration, and regulation phosphorylates p100 and promotes partial processing
of apoptosis, among others. [11,53] of p100 and yielding of p52. Consequent p52: RelB
dimmer then translocates into nucleus and regulate the
Classical and alternative NF-kB signaling transcription of its target genes.
pathways
Previous studies have established two well-defined DNA damage-induced NF-kB signaling
NF-kB activation signaling pathways initiated from pathway
membrane-bound receptors, the so-called “classical”
and “alternative” pathways. [54] The classical NF-kB DNA-damaging agents also activate NF-kB in a
pathway depends on activity of the IKK (IkB kinase) canonical IKK complex-dependent fashion. However,
kinase complex, which is composed of IKKα, IKKβ and in contrast to classical or alternative NF-kB signaling
IKKγ/NEMO. Upon activation of the IKK complex, the pathways, this genotoxic signaling cascade is initiated
IKKβ subunit directly phosphorylates NF-kB-associated in the nucleus instead of via membrane-bound
IkBα, leading to its proteasomal degradation and release receptors. In the following section, we will discuss
of p65/p50 heterodimer. Free NF-kB then translocates the detailed molecular signaling events mediating this
into the nucleus and regulate gene transcription. The retrograde signaling pathway [Figure 1]. [6,14]
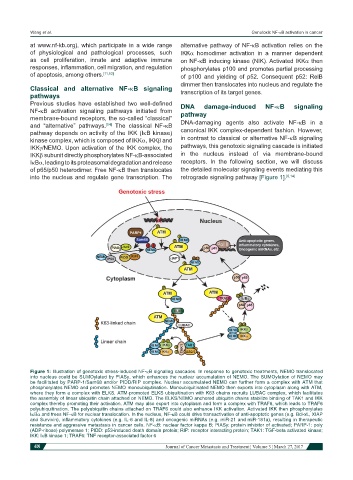
Figure 1: Illustration of genotoxic stress-induced NF-kB signaling cascades. In response to genotoxic treatments, NEMO translocated
into nucleus could be SUMOylated by PIASy, which enhances the nuclear accumulation of NEMO. The SUMOylation of NEMO may
be facilitated by PARP-1/Sam68 and/or PIDD/RIP complex. Nuclear accumulated NEMO can further form a complex with ATM that
phosphorylates NEMO and promotes NEMO monoubiqutination. Monoubiquitinated NEMO then exports into cytoplasm along with ATM,
where they form a complex with ELKS. ATM-promoted ELKS ubiquitination with K63 chains recruits LUBAC complex, which facilitates
the assembly of linear ubiquitin chain attached on NEMO. The ELKS/NEMO anchored ubiquitin chains stabilize binding of TAK1 and IKK
complex thereby promoting their activation. ATM may also export into cytoplasm and form a complex with TRAF6, which leads to TRAF6
polyubiquitination. The polyubiquitin chains attached on TRAF6 could also enhance IKK activation. Activated IKK then phosphorylates
IkBα and frees NF-kB for nuclear translocation. In the nucleus, NF-kB could drive transactivation of anti-apoptotic genes (e.g. Bcl-xL, XIAP
and Survivin), inflammatory cytokines (e.g. IL-6 and IL-8) and oncogenic miRNAs (e.g. miR-21 and miR-181a), resulting in therapeutic
resistance and aggressive metastasis in cancer cells. NF-kB: nuclear factor kappa B; PIASy: protein inhibitor of activated; PARP-1: poly
(ADP-ribose) polymerase 1; PIDD: p53-induced death domain protein; RIP: receptor interacting protein; TAK1: TGF-beta activated kinase;
IKK: IkB kinase 1; TRAF6: TNF receptor-associated factor 6
48 Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ March 27, 2017