Page 50 - Read Online
P. 50
Koukourakis et al. J Cancer Metastasis Treat 2022;8:38 https://dx.doi.org/10.20517/2394-4722.2022.43 Page 11 of 23
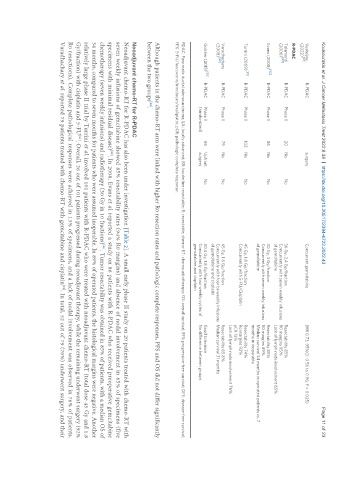
Vesteijne R-PDAC surgery Concurrent gemcitabine (HR 0.73, 95%CI: 0.56 to 0.96; P = 0.025)
[87]
(2022)
R-PDAC
Talamonti R-PDAC Phase II 20 No No 36 Gy, 2.4 Gy/fraction Resectability 85%
(2006) [89] Concurrently with seven weekly infusions R0 margins 95%
of gemcitabine Lack of lymph node involvement 65%
[90]
Evans (2008) R-PDAC Phase II 86 No No 30 Gy, 3 Gy/fraction Resectability 85%
Concurrently with seven weekly infusions R0 margins 89%
of gemcitabine Median survival 34 months in operated patients vs. 7
months in inoperable
[91]
Turrini (2009) R-PDAC Phase II 102 No No 45 Gy, 1.8 Gy/fraction Resectability 74%
Concurrently with 5-FU/Cisplatin Ro margins 92%
pCR 13%
Lack of lymph node involvement 76%
Varadhachary R-PDAC Phase II 79 No No 45 Gy, 1.8 Gy/fraction Resectability 65.8%
(2008) [92] Concurrently with four bi-weekly infusions Median survival 31 months
of gemcitabine and cisplatin
[93]
Golcher (2015) R-PDAC Phase II 66 Upfront No 50.4 Gy, 1.8 Gy/fraction Good tolerance
(randomized) surgery Concurrently with four weekly cycles of No difference between groups
gemcitabine and cisplatin
PDAC: Pancreatic ductal adenocarcinoma; LA: locally advanced; BR: borderline resectable; R: resectable; chemo-RT: chemoradiotherapy; OS: overall survival; PFS: progression-free survival; DFS: disease-free survival;
FFX: 5-FU/leucovorin/irinotecan/oxaliplatin; pCR: pathologic complete response.
Although patients in the chemo-RT arm were linked with higher R0 resection rates and pathologic complete responses, PFS and OS did not differ significantly
between the two groups .
[88]
Neoadjuvant chemo-RT for R-PDAC
Neoadjuvant chemo-RT for R-PDAC has also been under investigation [Table 2]. A small early phase II study on 20 patients treated with chemo-RT with
seven weekly infusions of gemcitabine showed 85% resectability rates (94% R0 margins) and absence of nodal involvement in 65% of specimens (five
specimens with minimal residual disease) . In 2008, Evans et al. reported a study on 86 patients with R-PDAC who received preoperative gemcitabine
[89]
[90]
chemotherapy (seven weekly infusions) and radiotherapy (30 Gy in 10 fractions) . Tumor resectability was obtained in 85% of patients, with a median OS of
34 months, compared to seven months for patients who were assumed inoperable. In 89% of operated patients, the histological margins were negative. Another
relatively large phase II trial by Turrini et al. involved 101 patients with R-PDAC who were treated with neoadjuvant chemo-RT (total dose 45 Gy and 1.8
Gy/fraction) with cisplatin and 5-FU . Overall, 26 out of 101 patients progressed during neoadjuvant therapy, while the remaining underwent surgery (92%
[91]
R0 resections). Complete pathological responses were achieved in 13% of specimens, and a lack of nodal involvement was observed in 76% of patients.
Varadhachary et al. reported 79 patients treated with chemo-RT with gemcitabine and cisplatin . In total, 52 out of 79 (70%) underwent surgery, and their
[92]