Page 53 - Read Online
P. 53
Page 14 of 23 Koukourakis et al. J Cancer Metastasis Treat 2022;8:38 https://dx.doi.org/10.20517/2394-4722.2022.43
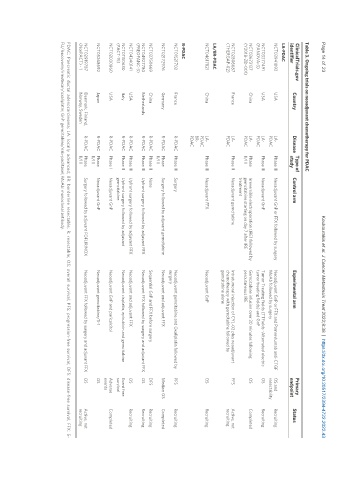
Table 3. Ongoing trials on neoadjuvant chemotherapy for PDAC
ClinicalTrials.gov Country Disease Type of Control arm Experimental arm Primary Status
identifier study endpoint
LA-PDAC
NCT03941093 USA LA- Phase III Neoadjuvant GnP or FFX followed by surgery Neoadjuvant GnP or FFX and Pamrevlumab anti-CTGF OS and Recruiting
PDAC MoAb followed by surgery resectablity
NCT03377491 USA LA- Phase III Neoadjuvant GnP Tumor Treating Fields (TTFields -Alternated electric OS Recruiting
(PANOVA-3) PDAC tumor treating fields) and GnP
NCT03673137 China LA- Phase Irreversible electroporation (IRE) followed by Gemcitabine infusion over 30 minutes following OS Completed
(Y2018-ZD-001) PDAC II/III gemcitabine starting on day 7 after IRE percutaneous IRE
treatment
NCT02806687 France LA- Phase II Neoadjuvant gemcitabine Intratumoral injection of CYL-02 plus neoadjuvant PFS Active, not
(THERGAP-02) PDAC chemotherapy with gemcitabine followed by recruiting
gemcitabine alone
LA/BR-PDAC
NCT04617821 China LA- Phase III Neoadjuvant FFX Neoadjuvant GnP OS Recruiting
PDAC
BR-
PDAC
R-PDAC
NCT01521702 France R-PDAC Phase III Surgery Neoadjuvant gemcitabine and Oxaliplatin followed by PFS Recruiting
surgery
NCT02172976 Germany R-PDAC Phase Surgery followed by adjuvant gemcitabine Neoadjuvant and adjuvant FFX Median OS Completed
II/III
NCT03750669 China R-PDAC Phase II None Sequential GnP and FFX before surgery DFS Recruiting
NCT04927780 Netherlands R-PDAC Phase III Upfront surgery followed by adjuvant FFX Neoadjuvant FFX followed by surgery and adjuvant FFX OS Recruiting
(PREOPANC-3)
NCT04340141 USA R-PDAC Phase III Upfront surgery followed by adjuvant FFX Neoadjuvant and adjuvant FFX OS Recruiting
NCT01150630 Italy R-PDAC Phase II Upfront surgery followed by adjuvant Neoadjuvant cisplatin, epirubicin and gemcitabine Event free
(PACT-15) gemcitabine survival
NCT02030860 USA R-PDAC Phase I Neoadjuvant GnP Neoadjuvant GnP and paricalcitol Adverse Completed
events
NCT05268692 Japan R-PDAC Phase Neoadjuvant GnP Neoadjuvant gemcitabine/S-1 OS
II/III
NCT02919787 Denmark, Finland, R-PDAC Phase Surgery followed by adjuvant FOFLIRINOX Neoadjuvant FFX followed by surgery and adjuvant FFX OS Active, not
(NorPACT) - 1 Norway, Sweden II/III recruiting
PDAC: Pancreatic ductal adenocarcinoma; LA: locally advanced; BR: borderline resectable; R: resectable; OS: overall survival; PFS: progression-free survival; DFS: disease-free survival; FFX: 5-
FU/leucovorin/irinotecan/oxaliplatin; GnP: gemcitabine/nab-paclitaxel; MoAb: monoclonal antibody.