Page 49 - Read Online
P. 49
Page 10 of 23 Koukourakis et al. J Cancer Metastasis Treat 2022;8:38 https://dx.doi.org/10.20517/2394-4722.2022.43
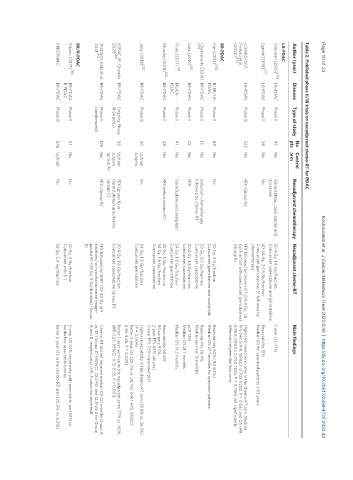
Table 2. Published phase II/III trials on neoadjuvant chemo-RT for PDAC
No Control
Author (year) Disease Type of study Neoadjuvant chemotherapy Neoadjuvant chemo-RT Main findings
pts arm
LA-PDAC
Sherman (2015) [76] LA-PDAC Phase II 45 No Gemcitabine, capecitabine and 50.4 Gy, 1.8 Gy/fraction 1-year OS: 71%
docetaxel Concurrent capecitabine and gemcitabine
Eguchi (2018) [77] LA-PDAC Phase II 34 No No 40-54 Gy, 2-1.8 Gy/fraction Resectability 15%
Concurrent gemcitabine/S1 followed by Median OS for operated patients 3.63 years
chemotherapy
CONKO-007; LA-PDAC Phase III 525 No FFX (Group A) FFX followed by chemo-RT (50.4 Gy, 1.8 Higher R0 resections rates in the chemo-RT arm. Median
Fietkau et al. Gy/fraction with concurrent gemcitabine) PFS (HR 0.919, 95%CI: 0.702-1.203, P = 0.54) and OS (HR
[88]
(2022) (Group B) 0.964, 95%CI: 0.760-1225, P = 0.766) not significantly
different between the two arms
BR-PDAC
[78]
Kim (2013) R/BR/LA- Phase II 68 No No 30 Gy, 2 Gy/fraction Resectability 63% (R0 84%)
PDAC Concurrent gemcitabine and oxaliplatin Median OS 27.1 months for operated patients
Chakraborty (2014) BR-PDAC Phase II 13 No Induction chemotherapy 50 Gy, 2.5 Gy/fraction Resectability 38.4%
[79]
followed by chemo-RT Concurrent capecitabine Median survival 13 months
[80]
Katz (2016) BR-PDAC Phase II 22 No FFX 50.4 Gy, 1.8 Gy/fraction pCR 13%
Concurrent capecitabine Median OS 21.7 months
[81]
Fiore (2017) BR/LA- Phase II 41 No Gemcitabine and oxaliplatin 54 Gy, 1.8 Gy/fraction Median OS 19.2 months
PDAC Concurrent gemcitabine
[82]
Murphy (2018) BR-PDAC Phase II 48 No FFX before chemo-RT 25 Gy, 5 Gy/fraction or Resectability 66.6%
54 Gy, 1.8 Gy/fraction R0 surgery 97%
Concurrent capecitabine 2-year PFS 43% (all pts)
2-year PFS 72% (operated pts)
[83]
Jang (2018) BR-PDAC Phase III 50 Upfront No 54 Gy, 1.8 Gy/fraction Higher resectability in the chemo-RT arm (51.8% vs. 26.1%)
surgery Concurrent gemcitabine (P = 0.004)
Better 2-year OS (30.7% vs. 26.1%) (HR 1.495, 95%CI:
0.66-3.36, P = 0.028)
ESPAC-5F; Ghaneh BR-PDAC Ongoing Phase 88 Upfront FFX (group B) or 50.4 Gy, 1.8 Gy/fraction Better 1-year survival in the neoadjuvant arms 77% vs. 40%
[46]
2020 III (4 arms) surgery Gemcitabine/capecitabine Concurrent capecitabine (group D) (HR 0.27, 95%CI: 0.13-0.55, P < 0.001)
(group A) (group C)
A021501; Katz et al. BR-PDAC Phase II 126 No FFX (Group A) FFX followed by SBRT (33-40 Gy in 5 Chemo-RT did not improve median OS (31 months Group A
2021 [84] (randomized) fractions) or hypofractionated image- vs. 17.1 Group B) (95%CI: 22.2-NE and 12.8-24.4 for Group
guided RT (25 Gy, 5 Gy/fraction) (Group A and B, respectively) (HR, P value not reported)
B)
BR/R-PDAC
Okano (2017) [85] BR-PDAC Phase II 57 No No 30 Gy, 3 Gy/fraction 2-year OS 83% in patients with resectable, and 58% in
R-PDAC Concurrent with S-1 borderline resectable tumors
PREOPANC; BR-PDAC Phase III 246 Upfront No 36 Gy, 2.4 Gy/fraction Better 5-year OS in the chemo-RT arm (20.5% vs. 6.5%)