Page 54 - Read Online
P. 54
Koukourakis et al. J Cancer Metastasis Treat 2022;8:38 https://dx.doi.org/10.20517/2394-4722.2022.43 Page 15 of 23
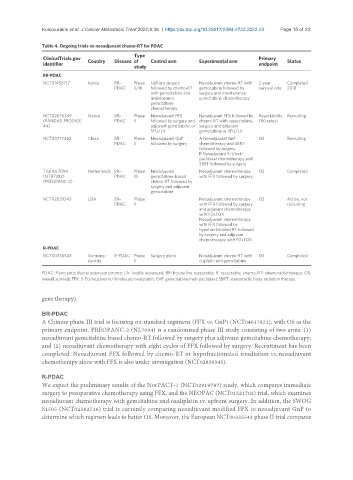
Table 4. Ongoing trials on neoadjuvant chemo-RT for PDAC
Type
ClinicalTrials.gov Primary
identifier Country Disease of Control arm Experimental arm endpoint Status
study
BR-PDAC
NCT01458717 Korea BR- Phase Upfront surgery Neoadjuvant chemo-RT with 2-year Completed
PDAC II/III followed by chemo-RT gemcitabine followed by survival rate 2018
with gemcitabine and surgery and maintenance
maintenance gemcitabine chemotherapy
gemcitabine
chemotherapy
NCT02676349 France BR- Phase Neoadjuvant FFX Neoadjuvant FFX followed by Resectability Recruiting
(PANDAS-PRODIGE PDAC II followed by surgery and chemo-RT with capecitabine, (R0 rates)
44) adjuvant gemcitabine or surgery and adjuvant
5FU/LV gemcitabine or 5FU/LV
NCT03777462 China BR- Phase Neoadjuvant GnP A Neoadjuvant GnP OS Recruiting
PDAC II followed by surgery chemotherapy and SBRT
followed by surgery
B Neoadjuvant S-1/nab-
paclitaxel chemotherapy and
SBRT followed by surgery
Trial NL7094 Netherlands BR- Phase Neoadjuvant Neoadjuvant chemotherapy OS Completed
(NTR7292) PDAC III gemcitabine-based with FFX followed by surgery
(PREOPANC-2) chemo-RT followed by
surgery and adjuvant
gemcitabine
NCT02839343 USA BR- Phase Neoadjuvant chemotherapy OS Active, not
PDAC II with FFX followed by surgery recruiting
and adjuvant chemotherapy
with FOLFOX
Neoadjuvant chemotherapy
with FFX followed by
hypofractionated RT followed
by surgery and adjuvant
chemotherapy with FOLFOX
R-PDAC
NCT00335543 Germany - R-PDAC Phase Surgery alone Neoadjuvant chemo-RT with OS Completed
Austria II cisplatin and gemcitabine
PDAC: Pancreatic ductal adenocarcinoma; LA: locally advanced; BR: borderline resectable; R: resectable; chemo-RT: chemoradiotherapy; OS:
overall survival; FFX: 5-FU/leucovorin/irinotecan/oxaliplatin; GnP: gemcitabine/nab-paclitaxel; SBRT: stereotactic body radiation therapy.
gene therapy).
BR-PDAC
A Chinese phase III trial is focusing on standard regimens (FFX vs. GnP) (NCT04617821), with OS as the
primary endpoint. PREOPANC-2 (NL7094) is a randomized phase III study consisting of two arms: (1)
neoadjuvant gemcitabine-based chemo-RT followed by surgery plus adjuvant gemcitabine chemotherapy;
and (2) neoadjuvant chemotherapy with eight cycles of FFX followed by surgery. Recruitment has been
completed. Neoadjuvant FFX followed by chemo-RT or hypofractionated irradiation vs. neoadjuvant
chemotherapy alone with FFX is also under investigation (NCT02839343).
R-PDAC
We expect the preliminary results of the NorPACT-1 (NCT02919787) study, which compares immediate
surgery to preoperative chemotherapy using FFX, and the NEOPAC (NCT01521702) trial, which examines
neoadjuvant chemotherapy with gemcitabine and oxaliplatin vs. upfront surgery. In addition, the SWOG
S1505 (NCT02562716) trial is currently comparing neoadjuvant modified FFX to neoadjuvant GnP to
determine which regimen leads to better OS. Moreover, the European NCT00335543 phase II trial compares