Page 55 - Read Online
P. 55
Page 16 of 23 Koukourakis et al. J Cancer Metastasis Treat 2022;8:38 https://dx.doi.org/10.20517/2394-4722.2022.43
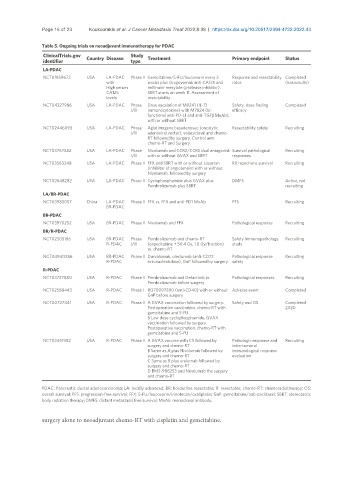
Table 5. Ongoing trials on neoadjuvant immunotherapy for PDAC
ClinicalTrials.gov Country Disease Study Treatment Primary endpoint Status
identifier type
LA-PDAC
NCT01959672 USA LA-PDAC Phase II Gemcitabine/5-FU/leucovorin every 3 Response and resectability Completed
with weeks plus Oregovomab anti-CA125 and rates (has results)
High serum nelfinavir mesylate (protease inhibitor).
CA125 SBRT starts on week 11. Assessment of
levels resectability
NCT04327986 USA LA-PDAC Phase Dose escalation of M9241 (IL-12 Safety, dose finding Completed
I/II immunocytokine) with M7824 (bi- efficacy
functional anti-PD-L1 and anti-TGFβ ΜοΑb),
with or without SBRT
NCT02446093 USA LA-PDAC Phase Aglatimagene besadenovec (oncolytic Resectability safety Recruiting
I/II adenoviral vector), valacyclovir and chemo-
RT followed by surgery. Control arm:
chemo-RT and Surgery
NCT03767582 USA LA-PDAC Phase Nivolumab and CCR2/CCR5 dual antagonist Survival pathological Recruiting
I/II with or without GVAX and SBRT responses
NCT03563248 USA LA-PDAC Phase II FFX and SBRT with or without Losartan R0 resections survival Recruiting
(inhibitor of angiotensin) with or without
Nivolumab, followed by surgery
NCT02648282 USA LA-PDAC Phase II Cyclophosphamide plus GVAX plus DMFS Active, not
Pembrolizumab plus SBRT recruiting
LA/BR-PDAC
NCT03983057 China LA-PDAC Phase II FFX vs. FFX and anti-PD1 MoAb PFS Recruiting
BR-PDAC
BR-PDAC
NCT03970252 USA BR-PDAC Phase II Nivolumab and FFX Pathological response Recruiting
BR/R-PDAC
NCT02305186 USA BR-PDAC Phase Pembrolizumab and chemo-RT Safety immunopathology Recruiting
R-PDAC I/II (capecitabine + 50.4 Gy, 1.8 Gy/fraction) study
vs. chemo-RT
NCT04940286 USA BR-PDAC Phase II Durvalumab, oleclumab (anti-CD73 Pathological response Recruiting
R-PDAC ectonucleotidase), GnP followed by surgery safety
R-PDAC
NCT03727880 USA R-PDAC Phase II Pembrolizumab and Defactinib vs. Pathological responses Recruiting
Pembrolizumab before surgery
NCT02588443 USA R-PDAC Phase I RO70097890 (anti-CD40) with or without Adverse event Completed
GnP before surgery
NCT00727441 USA R-PDAC Phase II A GVAX vaccination followed by surgery. Safety and OS Completed
Postoperative vaccination, chemo-RT with 2020
gemcitabine and 5-FU
B Low dose cyclophosphamide, GVAX
vaccination followed by surgery.
Postoperative vaccination, chemo-RT with
gemcitabine and 5-FU
NCT02451982 USA R-PDAC Phase II A GVAX vaccine with CY followed by Pathologic response and Recruiting
surgery and chemo-RT intra-tumoral
B Same as A plus Nivolumab followed by immunological response
surgery and chemo-RT evaluation
C Same as B plus urelumab followed by
surgery and chemo-RT
D BMS-986253 and Nivolumab the surgery
and chemo-RT
PDAC: Pancreatic ductal adenocarcinoma; LA: locally advanced; BR: borderline resectable; R: resectable; chemo-RT: chemoradiotherapy; OS:
overall survival; PFS: progression-free survival; FFX: 5-FU/leucovorin/irinotecan/oxaliplatin; GnP: gemcitabine/nab-paclitaxel; SBRT: stereotactic
body radiation therapy; DMFS: distant metastasis free survival; MoAb: monoclonal antibody.
surgery alone to neoadjuvant chemo-RT with cisplatin and gemcitabine.