Page 50 - Read Online
P. 50
Farlow et al. J Cancer Metastasis Treat 2019;5:18 I http://dx.doi.org/10.20517/2394-4722.2018.100 Page 3 of 10
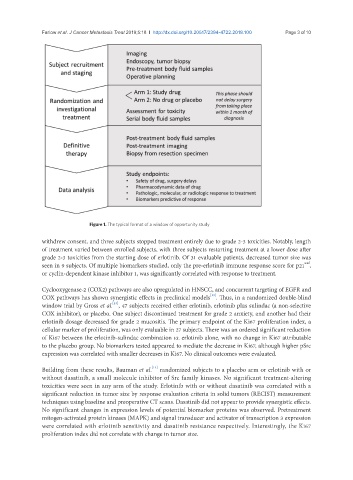
Figure 1. The typical format of a window of opportunity study
withdrew consent, and three subjects stopped treatment entirely due to grade 2-3 toxicities. Notably, length
of treatment varied between enrolled subjects, with three subjects restarting treatment at a lower dose after
grade 2-3 toxicities from the starting dose of erlotinib. Of 31 evaluable patients, decreased tumor size was
waf
seen in 9 subjects. Of multiple biomarkers studied, only the pre-erlotinib immune response score for p21 ,
or cyclin-dependent kinase inhibitor 1, was significantly correlated with response to treatment.
Cyclooxygenase-2 (COX2) pathways are also upregulated in HNSCC, and concurrent targeting of EGFR and
[13]
COX pathways has shown synergistic effects in preclinical models . Thus, in a randomized double-blind
[13]
window trial by Gross et al. , 47 subjects received either erlotinib, erlotinib plus sulindac (a non-selective
COX inhibitor), or placebo. One subject discontinued treatment for grade 2 anxiety, and another had their
erlotinib dosage decreased for grade 2 mucositis. The primary endpoint of the Ki67 proliferation index, a
cellular marker of proliferation, was only evaluable in 27 subjects. There was an ordered significant reduction
of Ki67 between the erlotinib-sulindac combination vs. erlotinib alone, with no change in Ki67 attributable
to the placebo group. No biomarkers tested appeared to mediate the decrease in Ki67, although higher pSrc
expression was correlated with smaller decreases in Ki67. No clinical outcomes were evaluated.
[14]
Building from these results, Bauman et al. randomized subjects to a placebo arm or erlotinib with or
without dasatinib, a small molecule inhibitor of Src family kinases. No significant treatment-altering
toxicities were seen in any arm of the study. Erlotinib with or without dasatinib was correlated with a
significant reduction in tumor size by response evaluation criteria in solid tumors (RECIST) measurement
techniques using baseline and preoperative CT scans. Dasatinib did not appear to provide synergistic effects.
No significant changes in expression levels of potential biomarker proteins was observed. Pretreatment
mitogen-activated protein kinases (MAPK) and signal transducer and activator of transcription 3 expression
were correlated with erlotinib sensitivity and dasatinib resistance respectively. Interestingly, the Ki67
proliferation index did not correlate with change in tumor size.