Page 61 - Read Online
P. 61
Page 6 of 14 Anand et al. J Cancer Metastasis Treat 2019;5:6 I http://dx.doi.org/10.20517/2394-4722.2018.98
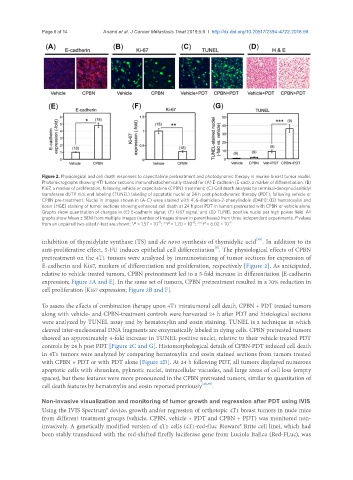
Figure 2. Physiological and cell death responses to capecitabine pretreatment and photodynamic therapy in murine breast tumor model.
Photomicrographs showing 4T1 tumor sections immunohistochemically stained for (A) E-cadherin (E-cad), a marker of differentiation; (B)
Ki67, a marker of proliferation, following vehicle or capecitabine (CPBN) treatment; (C) Cell death analysis by terminal-deoxynucleoitidyl
transferase dUTP nick end labeling (TUNEL) labeling of apoptotic nuclei at 24 h post photodynamic therapy (PDT), following vehicle or
CPBN pre-treatment. Nuclei in images shown in (A-C) were stained with 4’,6-diamidino-2-phenylindole (DAPI); (D) hematoxylin and
eosin (H&E) staining of tumor sections showing enhanced cell death at 24 h post PDT in tumors pretreated with CPBN or vehicle alone.
Graphs show quantitation of changes in (E) E-cadherin signal; (F) Ki67 signal; and (G) TUNEL positive nuclei per high power field. All
graphs show Mean ± SEM from multiple images (number of images shown in parentheses) from three independent experiments. P values
-11
-8
from an unpaired two-sided t-test are shown: *P = 1.57 × 10 ; **P = 1.20 × 10 ; ***P = 6.02 × 10 -9
[38]
inhibition of thymidylate synthase (TS) and de novo synthesis of thymidylic acid . In addition to its
[9]
anti-proliferative effect, 5-FU induces epithelial cell differentiation . The physiological effects of CPBN
pretreatment on the 4T1 tumors were analyzed by immunostaining of tumor sections for expression of
E-cadherin and Ki67, markers of differentiation and proliferation, respectively [Figure 2]. As anticipated,
relative to vehicle treated tumors, CPBN pretreatment led to a 5-fold increase in differentiation [E-cadherin
expression; Figure 2A and E]. In the same set of tumors, CPBN pretreatment resulted in a 70% reduction in
cell proliferation [Ki67 expression; Figure 2B and F].
To assess the effects of combination therapy upon 4T1 intratumoral cell death, CPBN + PDT treated tumors
along with vehicle- and CPBN-treatment controls were harvested 24 h after PDT and histological sections
were analyzed by TUNEL assay and by hematoxylin and eosin staining. TUNEL is a technique in which
cleaved inter-nucleosomal DNA fragments are enzymatically labeled in dying cells. CPBN pretreated tumors
showed an approximately 4-fold increase in TUNEL positive nuclei, relative to their vehicle treated PDT
controls by 24 h post PDT [Figure 2C and G]. Histomorphological details of CPBN-PDT induced cell death
in 4T1 tumors were analyzed by comparing hematoxylin and eosin stained sections from tumors treated
with CPBN + PDT or with PDT alone [Figure 2D]. At 24 h following PDT, all tumors displayed numerous
apoptotic cells with shrunken, pyknotic nuclei, intracellular vacuoles, and large areas of cell loss (empty
spaces), but these features were more pronounced in the CPBN pretreated tumors, similar to quantitation of
cell death features by hematoxylin and eosin reported previously [25,39] .
Non-invasive visualization and monitoring of tumor growth and regression after PDT using IVIS
Using the IVIS Spectrum® device, growth and/or regression of orthotopic 4T1 breast tumors in nude mice
from different treatment groups (vehicle, CPBN, vehicle + PDT and CPBN + PDT) was monitored non-
invasively. A genetically modified version of 4T1 cells (4T1-red-fluc Bioware® Brite cell line), which had
been stably transduced with the red-shifted firefly luciferase gene from Luciola Italica (Red-FLuc), was