Page 80 - Read Online
P. 80
Pippione et al. Steroidogenic enzymes in prostate cancer
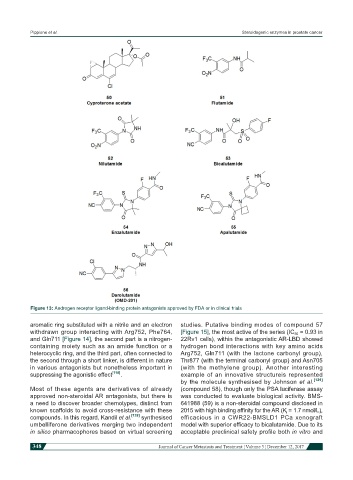
Figure 13: Androgen receptor ligand-binding protein antagonists approved by FDA or in clinical trials
aromatic ring substituted with a nitrile and an electron studies. Putative binding modes of compound 57
withdrawn group interacting with Arg752, Phe764, [Figure 15], the most active of the series (IC 50 = 0.93 in
and Gln711 [Figure 14], the second part is a nitrogen- 22Rv1 cells), within the antagonistic AR-LBD showed
containing moiety such as an amide function or a hydrogen bond interactions with key amino acids
heterocyclic ring, and the third part, often connected to Arg752, Gln711 (with the lactone carbonyl group),
the second through a short linker, is different in nature Thr877 (with the terminal carbonyl group) and Asn705
in various antagonists but nonetheless important in (with the methylene group). Another interesting
suppressing the agonistic effect [118] . example of an innovative structureis represented
by the molecule synthesised by Johnson et al. [129]
Most of these agents are derivatives of already (compound 58), though only the PSA luciferase assay
approved non-steroidal AR antagonists, but there is was conducted to evaluate biological activity. BMS-
a need to discover broader chemotypes, distinct from 641988 (59) is a non-steroidal compound disclosed in
known scaffolds to avoid cross-resistance with these 2015 with high binding affinity for the AR (K i = 1.7 nmol/L),
compounds. In this regard, Kandil et al. [128] synthesised efficacious in a CWR22-BMSLD1 PCa xenograft
umbelliferone derivatives merging two independent model with superior efficacy to bicalutamide. Due to its
in silico pharmacophores based on virtual screening acceptable preclinical safety profile both in vitro and
348 Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ December 12, 2017