Page 73 - Read Online
P. 73
Pippione et al. Steroidogenic enzymes in prostate cancer
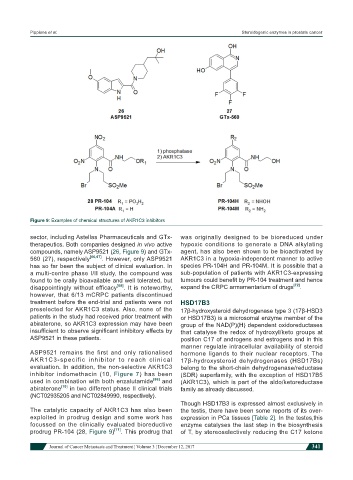
Figure 9: Examples of chemical structures of AKR1C3 inhibitors
sector, including Astellas Pharmaceuticals and GTx- was originally designed to be bioreduced under
therapeutics. Both companies designed in vivo active hypoxic conditions to generate a DNA alkylating
compounds, namely ASP9521 (26, Figure 9) and GTx- agent, has also been shown to be bioactivated by
560 (27), respectively [66,67] . However, only ASP9521 AKR1C3 in a hypoxia-independent manner to active
has so far been the subject of clinical evaluation. In species PR-104H and PR-104M. It is possible that a
a multi-centre phase I/II study, the compound was sub-population of patients with AKR1C3-expressing
found to be orally bioavailable and well tolerated, but tumours could benefit by PR-104 treatment and hence
disappointingly without efficacy [68] . It is noteworthy, expand the CRPC armamentarium of drugs [72] .
however, that 6/13 mCRPC patients discontinued
treatment before the end-trial and patients were not HSD17B3
preselected for AKR1C3 status. Also, none of the 17β-hydroxysteroid dehydrogenase type 3 (17β-HSD3
patients in the study had received prior treatment with or HSD17B3) is a microsomal enzyme member of the
abiraterone, so AKR1C3 expression may have been group of the NAD(P)(H) dependent oxidoreductases
insufficient to observe significant inhibitory effects by that catalyse the redox of hydroxyl/keto groups at
ASP9521 in these patients. position C17 of androgens and estrogens and in this
manner regulate intracellular availability of steroid
ASP9521 remains the first and only rationalised hormone ligands to their nuclear receptors. The
AKR1C3-specific inhibitor to reach clinical 17β-hydroxysteroid dehydrogenases (HSD17Bs)
evaluation. In addition, the non-selective AKR1C3 belong to the short-chain dehydrogenase/reductase
inhibitor indomethacin (10, Figure 7) has been (SDR) superfamily, with the exception of HSD17B5
used in combination with both enzalutamide [69] and (AKR1C3), which is part of the aldo/ketoreductase
abiraterone [70] in two different phase II clinical trials family as already discussed.
(NCT02935205 and NCT02849990, respectively).
Though HSD17B3 is expressed almost exclusively in
The catalytic capacity of AKR1C3 has also been the testis, there have been some reports of its over-
exploited in prodrug design and some work has expression in PCa tissues [Table 2]. In the testes,this
focussed on the clinically evaluated bioreductive enzyme catalyses the last step in the biosynthesis
prodrug PR-104 (28, Figure 9) [71] . This prodrug that of T, by stereoselectively reducing the C17 ketone
Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ December 12, 2017 341