Page 230 - Read Online
P. 230
Shibata Living donor liver transplantation anastomotic stenosis interventional radiology balloon dilatation
IR FOR HVOO challenge for surgeons.
Vascular complications after liver transplantation Procedures
include occlusion/stenosis at the site of anastomosis The approach to the hepatic vein is made through
of hepatic artery, portal vein and hepatic vein. transjugular or transhepatic method. After passage of
Although HVOO is an uncommon complication after liver the catheter through the stenotic segment of the hepatic
transplantation, it is still an important cause of graft vein, venography and manometry; measurement of
[2]
failures after liver transplantation . The incidence of venous pressure of proximal and distal sides of the
HVOO after orthotropic liver transplantation is reported stenosis and the pressure gradient across the stricture
to be about 1% and that after LDLT is reported to be is performed. Patients with a pressure gradient of more
about 2-4% [11,12] . This is because an anastomotic orifice than 3 mmHg are considered to have significant outflow
is small and the grafts grow in LDLT. The causes of obstruction and are candidates for balloon dilatation.
HVOO were stretching, twist and compression of
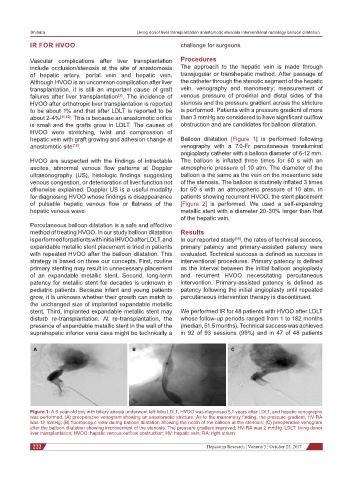
hepatic vein with graft growing and adhesion change at Balloon dilatation [Figure 1] is performed following
anastomotic site [13] . venography with a 7.0-Fr percutaneous transluminal
angioplasty catheter with a balloon diameter of 6-12 mm.
HVOO are suspected with the findings of intractable The balloon is inflated three times for 60 s with an
ascites, abnormal venous flow patterns at Doppler atmospheric pressure of 10 atm. The diameter of the
ultrasonography (US), histologic findings suggesting balloon is the same as the vein on the mesenteric side
venous congestion, or deterioration of liver function not of the stenosis. The balloon is routinely inflated 3 times
otherwise explained. Doppler US is a useful modality for 60 s with an atmospheric pressure of 10 atm. In
for diagnosing HVOO whose findings is disappearance patients showing recurrent HVOO, the stent placement
of pulsatile hepatic venous flow or flatness of the [Figure 2] is performed. We used a self-expanding
hepatic venous wave. metallic stent with a diameter 20-30% larger than that
of the hepatic vein.
Percutaneous balloon dilatation is a safe and effective
method of treating HVOO. In our study balloon dilatation Results
is performed for patients with initial HVOO after LDLT, and In our reported study [14] , the rates of technical success,
expandable metallic stent placement is tried in patients primary patency and primary-assisted patency were
with repeated HVOO after the balloon dilatation. This evaluated. Technical success is defined as success in
strategy is based on three our concepts. First, routine interventional procedures. Primary patency is defined
primary stenting may result in unnecessary placement as the interval between the initial balloon angioplasty
of an expandable metallic stent. Second, long-term and recurrent HVOO necessitating percutaneous
patency for metallic stent for decades is unknown in intervention. Primary-assisted patency is defined as
pediatric patients. Because infant and young patients patency following the initial angioplasty until repeated
grow, it is unknown whether their growth can match to percutaneous intervention therapy is discontinued.
the unchanged size of implanted expandable metallic
stent. Third, implanted expandable metallic stent may We performed IR for 48 patients with HVOO after LDLT
disturb re-transplantation. At re-transplantation, the whose follow-up periods ranged from 1 to 182 months
presence of expandable metallic stent in the wall of the (median, 51.5 months). Technical success was achieved
suprahepatic inferior vena cava might be technically a in 92 of 93 sessions (99%) and in 47 of 48 patients
A B C
Figure 1: A 6-year-old boy with biliary atresia underwent left-lobe LDLT, HVOO was diagnosed 5.1 years after LDLT, and hepatic venography
was performed. (A) preoperative venogram showing an anastomotic stricture. As to the manometry finding, the pressure gradient, HV-RA
was 12 mmHg; (B) fluoroscopic view during balloon dilatation showing the notch of the balloon at the stenosis; (C) preoperative venogram
after the balloon dilatation showing improvement of the stenosis. The pressure gradient improved; HV-RA was 2 mmHg. LDLT: living donor
liver transplantation; HVOO: hepatic venous outflow obstruction; HV: hepatic vein; RA: right atrium
222 Hepatoma Research ¦ Volume 3 ¦ October 25, 2017