Page 231 - Read Online
P. 231
Shibata Living donor liver transplantation anastomotic stenosis interventional radiology balloon dilatation
in patients with reduced-size liver transplantation or
A B
LDLT, the rate of PV complication can be higher (9-
14%) than in patients with conventional deceased donor
liver transplantation [7,15] . PV complications are divided
mainly into anastomotic PVS and portal vein thrombosis
(PVT) [16] . Anastomotic PVS can lead to graft failure if
not properly treated. The treatment options for PVS
after liver transplantation are surgical treatment and
percutaneous interventions, including percutaneous
balloon dilatation and stent placement. However,
C D surgical treatment of these complications has been
limited owing to technical difficulties or invasiveness.
Currently, the surgical treatment of PVS after liver
transplantation has been replaced by percutaneous
balloon dilatation and stent placement, because of
lower invasiveness and greater effectiveness.
PVS was clinically suspected with the following
findings: (1) clinical symptoms of portal hypertension,
such as ascites, splenomegaly, gastrointestinal tract
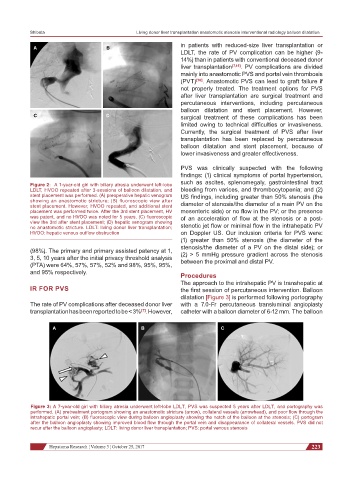
Figure 2: A 1-year-old girl with biliary atresia underwent left-lobe
LDLT, HVOO repeated after 3-sessions of balloon dilatation, and bleeding from varices, and thrombocytopenia; and (2)
stent placement was performed. (A) preoperative hepatic venogram US findings, including greater than 50% stenosis (the
showing an anastomotic stricture; (B) fluoroscopic view after
stent placement. However, HVOO repeated, and additional stent diameter of stenosis/the diameter of a main PV on the
placement was performed twice. After the 3rd stent placement, HV mesenteric side) or no flow in the PV; or the presence
was patent, and no HVOO was noted for 5 years. (C) fluoroscopic of an acceleration of flow at the stenosis or a post-
view the 3rd after stent placement; (D) hepatic venogram showing
no anastomotic stricture. LDLT: living donor liver transplantation; stenotic jet flow or minimal flow in the intrahepatic PV
HVOO: hepatic venous outflow obstruction on Doppler US. Our inclusion criteria for PVS were:
(1) greater than 50% stenosis (the diameter of the
stenosis/the diameter of a PV on the distal side); or
(98%). The primary and primary assisted patency at 1, (2) > 5 mmHg pressure gradient across the stenosis
3, 5, 10 years after the initial privacy threshold analysis between the proximal and distal PV.
(PTA) were 64%, 57%, 57%, 52% and 98%, 95%, 95%,
and 95% respectively. Procedures
The approach to the intrahepatic PV is transhepatic at
IR FOR PVS the first session of percutaneous intervention. Balloon
dilatation [Figure 3] is performed following portography
The rate of PV complications after deceased donor liver with a 7.0-Fr percutaneous transluminal angioplasty
transplantation has been reported to be < 3% . However, catheter with a balloon diameter of 6-12 mm. The balloon
[7]
A B C
Figure 3: A 7-year-old girl with biliary atresia underwent left-lobe LDLT, PVS was suspected 5 years after LDLT, and portography was
performed. (A) pretreatment portogram showing an anastomotic stricture (arrow), collateral vessels (arrowhead), and poor flow through the
intrahepatic portal vein; (B) fluoroscopic view during balloon angioplasty showing the notch of the balloon at the stenosis; (C) portogram
after the balloon angioplasty showing improved blood flow through the portal vein and disappearance of collateral vessels. PVS did not
recur after the balloon angioplasty; LDLT: living donor liver transplantation; PVS: portal venous stenosis
Hepatoma Research ¦ Volume 3 ¦ October 25, 2017 223