Page 207 - Read Online
P. 207
hepatic artery via a femoral artery puncture is used to with this combination and no diminishment of antitumor
selectively administer an anti-neoplastic agent directly to activity with advanced disease burden in the liver. However,
[3]
liver tumors. By endovascular venous cannulation, a unique due to the unavailability of TNFα for continued clinical testing
double balloon catheter (Delcath catheter) is inserted into the in the United States, melphalan has been the most widely
inferior vena cava (IVC) to capture the hepatic venous outflow used chemotherapeutic agent in current trials. Through these
from the liver. Using veno-venous bypass, the chemotherapy early studies of the operative technique for IHP, key elements
laden blood can be captured at the hepatic vein confluence and principles were noted and carried over to the minimally
and filtered before returning to the systemic circulation by invasive PHP technique in use today.
a central venous line. This novel treatment technique has
evolved from original operative liver isolation techniques, PHP was initially reported approximately 20 years ago by 2
[6]
which capitalized on the hepatic anatomy for inflow and centers. The largest study described by Ravikumar et al.
surgical outflow control in liver directed perfusion. [3] involved 28 patients who were treated with escalating doses
of doxorubicin or 5-fluorouracil. Through the catheter based
History and development approach, the chemotherapy was administered via a hepatic
The first use of hepatic perfusion was reported by Dr. Robert artery catheter and collected and filtered using veno-venous
Ausman in 1961 as a surgery resident at the Roswell Park bypass from the venous outflow of the liver. Concurrently,
[7]
Cancer Institute where he developed the technique. His a phase I study by Curley et al. was being performed in
initial studies were performed on animal models, and once patients with hepatocellular carcinoma. Similar to the early
the technique was standardized it was tested on 5 patients use of IHP, no long term follow-up data was published and
with different types of hepatic malignancies. Though there these studies were not continued at these centers. However,
was no long term follow-up and significant toxicity noted these studies described the potential use of this procedure
with the procedure, there was a therapeutic effect described and contributed to the refinement of its technical feasibility.
in 2 patients. This initial study helped lay the foundation
[3]
for isolated hepatic perfusion (IHP) which has been refined In 2005, the comprehensive evaluation of PHP was conducted
over 60 years. Multiple centers have evaluated IHP with as a phase I trial at the National Cancer Institute where 28
various chemotherapy agents, various tumor histologies, patients were treated with melphalan PHP, for 74 treatments
hyperthermic perfusion, and improved techniques. [4] in a dose escalation format. The overall radiographic response
rate was observed to be 30% (RECIST criteria), with rates as
With data from isolated limb perfusion by Lienard et al. in high as 50% in 10 patients with metastatic ocular melanoma.
[5]
1992, melphalan was initially tested in combination with Though transient hepatic toxicity and some hematologic
tumor necrosis factor alpha (TNFα). This regimen was used toxicity were observed, this study helped determine the
for IHP to treat liver disease. Early results at the National maximum tolerated dose of melphalan (3.0 mg/kg) and
Cancer Institute showed a 75% radiographic response rate established the groundwork for a multicenter trial. After
[8]
®
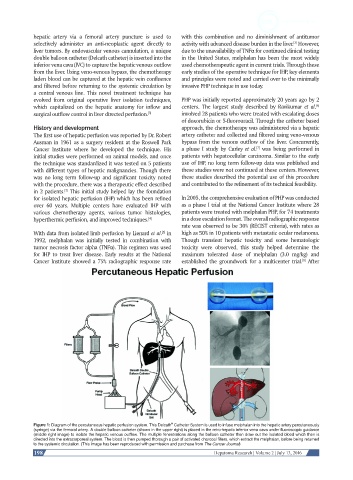
Figure 1: Diagram of the percutaneous hepatic perfusion system. This Delcath Catheter System is used to infuse melphalan into the hepatic artery percutaneously
(syringe) via the femoral artery. A double balloon catheter (shown in the upper right) is placed in the retro-hepatic inferior vena cava under fluoroscopic guidance
(middle right image) to isolate the hepatic venous outflow. The multiple fenestrations along the balloon catheter then draw out the isolated blood which then is
directed into the extracorporeal system. The blood is then pumped thorough a pair of activated charcoal filters, which extract the melphalan, before being returned
to the systemic circulation. (This image has been reproduced with permission and purchase from The Cancer Journal)
198 Hepatoma Research | Volume 2 | July 13, 2016