Page 109 - Read Online
P. 109
DISCUSSION sorafenib could be combined with other established anti-HBV
agents to treat CHB because the combined therapy with other
The antiviral effect of sorafenib on HBV gene expression mechanisms is expected to show improved efficacy.
indicates meaningful approaches to anti-HBV drugs. There
are several available agents for the treatment of CHB. Sorafenib is a medicine for HCC and HCC is mainly caused
[12]
These drugs, including immunomodulatory agents and by HBV infection. Therefore, we expect that sorafenib
nucleotide/nucleoside analogs, have high efficacy against will help block the progress of hepatitis B and prevent the
CHB, but there are several limitations including side effects, development of HCC. This might be a unique advantage as
[9]
tolerance, and drug resistance. These limitations have been an antiviral drug because other currently available anti-HBV
[10]
considered as problems to overcome for at least 10 years. agents don’t have oncomodulatory effects. In addition,
Currently, combined therapies of these drugs show improved targeting the host molecular signaling pathway is expected to
[11]
efficacy and lower drug resistance in CHB. However, other have less drug-resistance compared to nucleotide/nucleoside
drugs with new strategies for HBV replication have not been analogs, which targets viral polymerase with high genetic
[9]
approved yet. The development of more effective drugs for variation. The mechanism of the effect of sorafenib on
the management of CHB has proven to be challenging. Here, HBV gene expression should be elucidated with a variety of
sorafenib suppressed HBV gene expression by inhibiting the studies in vitro and in vivo. Especially, the effect of sorafenib
JNK signaling pathway. This new mechanism suggests another on anti-HBV drug-resistant strains should be investigated
possible approach to inhibit HBV replication. Furthermore, through combined therapy.
d
c
a b
e f g
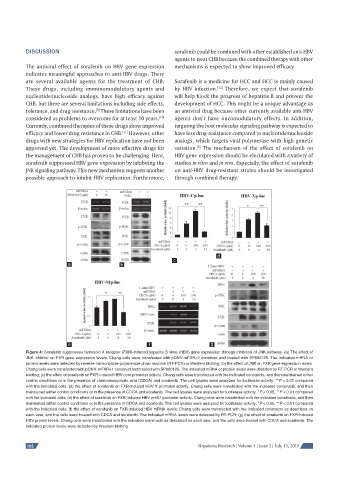
Figure 4: Sorafenib suppresses farnesoid X receptor (FXR)-induced hepatitis B virus (HBV) gene expression through inhibition of JNK pathway. (a) The effect of
JNK inhibitor on FXR gene expression levels. Chang cells were transfected with pCMV-mFXRα1 construct and treated with SP600125. The indicated mRNA or
protein levels were detected by reverse transcriptase-polymerase chain reaction (RT-PCR) or Western blotting; (b) the effect of JNK on FXR gene expression levels.
Chang cells were transfected with pCMV-mFXRα1 construct and treated with SP600125. The indicated mRNA or protein levels were detected by RT-PCR or Western
blotting; (c) the effect of sorafenib on FXR-induced HBV core promoter activity. Chang cells were transfected with the indicated constructs, and then maintained either
control conditions or in the presence of chenodeoxycholic acid (CDCA) and sorafenib. The cell lysates were analyzed for luciferase activity. **P < 0.01 compared
with the indicated cells; (d) the effect of sorafenib on FXR-induced HBV X promoter activity. Chang cells were transfected with the indicated constructs, and then
maintained either control conditions or in the presence of CDCA and sorafenib. The cell lysates were analyzed for luciferase activity. *P < 0.05, **P < 0.01 compared
with the indicated cells; (e) the effect of sorafenib on FXR-induced HBV preS1 promoter activity. Chang cells were transfected with the indicated constructs, and then
maintained either control conditions or in the presence of CDCA and sorafenib. The cell lysates were analyzed for luciferase activity. *P < 0.05, **P < 0.01 compared
with the indicated cells; (f) the effect of sorafenib on FXR-induced HBV mRNA levels. Chang cells were transfected with the indicated constructs as described on
each lane, and the cells were treated with CDCA and sorafenib. The indicated mRNA levels were detected by RT-PCR; (g) the effect of sorafenib on FXR-induced
HBV protein levels. Chang cells were transfected with the indicated constructs as described on each lane, and the cells were treated with CDCA and sorafenib. The
indicated protein levels were detected by Western blotting
102 Hepatoma Research | Volume 1 | Issue 2 | July 15, 2015