Page 26 - Read Online
P. 26
Allen et al. Hepatoma Res 2021;7:73 https://dx.doi.org/10.20517/2394-5079.2021.98 Page 7 of 15
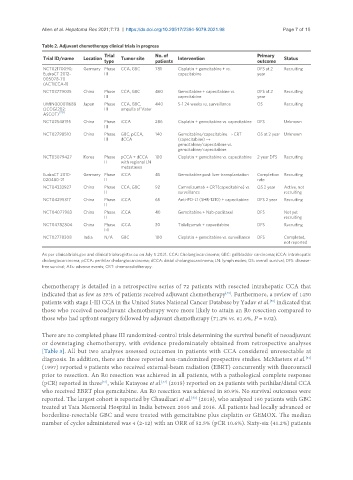
Table 2. Adjuvant chemotherapy clinical trials in progress
Trial No. of Primary
Trial ID/name Location Tumor site Intervention Status
type patients outcome
NCT02170090; Germany Phase CCA, GBC 781 Cisplatin + gemcitabine + vs. DFS at 2 Recruiting
EudraCT 2012- III capecitabine year
005078-70
(ACTICCA-1)
NCT03779035 China Phase CCA, GBC 460 Gemcitabine + capecitabine vs. DFS at 2 Recruiting
III capecitabine year
UMIN000011688 Japan Phase CCA, GBC, 440 S-1 24 weeks vs. surveillance OS Recruiting
(JCOG1202: III ampulla of Vater
ASCOT) [70]
NCT02548195 China Phase iCCA 286 Cisplatin + gemcitabine vs. capecitabine DFS Unknown
III
NCT02798510 China Phase GBC, pCCA, 140 Gemcitabine/capecitabine → CRT OS at 2 year Unknown
III dCCA (capecitabine) →
gemcitabine/capecitabine vs.
gemcitabine/capecitabine
NCT03079427 Korea Phase pCCA + dCCA 100 Cisplatin + gemcitabine vs. capecitabine 2 year DFS Recruiting
II with regional LN
metastases
EudraCT 2010- Germany Phase iCCA 45 Gemcitabine post liver transplantation Completion Recruiting
020480-21 II rate
NCT04333927 China Phase CCA, GBC 92 Camrelizumab + CRT(capecitabine) vs. OS 2 year Active, not
II surveillance recruiting
NCT04295317 China Phase iCCA 65 Anti-PD-L1 (SHR-1210) + capecitabine DFS 2 year Recruiting
II
NCT04077983 China Phase iCCA 40 Gemcitabine + Nab-paclitaxel DFS Not yet
II recruiting
NCT04782804 China Phase iCCA 30 Tislelizumab + capecitabine DFS Recruiting
I-II
NCT02778308 India N/A GBC 100 Cisplatin + gemcitabine vs. surveillance DFS Completed,
not reported
As per clinicaltrials.gov and clinicaltrialsregister.eu on July 5 2021. CCA: Cholangiocarcinoma; GBC: gallbladder carcinoma; iCCA: intrahepatic
cholangiocarcinoma; pCCA: perihilar cholangiocarcinoma; dCCA: distal cholangiocarcinoma; LN: lymph nodes; OS: overall survival; DFS: disease-
free survival; AEs: adverse events; CRT: chemoradiotherapy.
chemotherapy is detailed in a retrospective series of 72 patients with resected intrahepatic CCA that
indicated that as few as 35% of patients received adjuvant chemotherapy . Furthermore, a review of 1450
[49]
patients with stage I-III CCA in the United States National Cancer Database by Yadav et al. indicated that
[50]
those who received neoadjuvant chemotherapy were more likely to attain an R0 resection compared to
those who had upfront surgery followed by adjuvant chemotherapy (71.2% vs. 61.6%, P = 0.02).
There are no completed phase III randomized-control trials determining the survival benefit of neoadjuvant
or downstaging chemotherapy, with evidence predominately obtained from retrospective analyses
[Table 3]. All but two analyses assessed outcomes in patients with CCA considered unresectable at
diagnosis. In addition, there are three reported non-randomized prospective studies. McMasters et al.
[51]
(1997) reported 9 patients who received external-beam radiation (EBRT) concurrently with fluorouracil
prior to resection. An R0 resection was achieved in all patients, with a pathological complete response
(pCR) reported in three , while Katayose et al. (2015) reported on 24 patients with perihilar/distal CCA
[51]
[52]
who received EBRT plus gemcitabine. An R0 resection was achieved in 80.9%. No survival outcomes were
reported. The largest cohort is reported by Chaudhari et al. (2018), who analyzed 160 patients with GBC
[53]
treated at Tata Memorial Hospital in India between 2010 and 2016. All patients had locally advanced or
borderline-resectable GBC and were treated with gemcitabine plus cisplatin or GEMOX. The median
number of cycles administered was 4 (2-12) with an ORR of 52.5% (pCR 10.6%). Sixty-six (41.2%) patients