Page 31 - Read Online
P. 31
Page 10 of 15 Allen et al. Hepatoma Res 2021;7:73 https://dx.doi.org/10.20517/2394-5079.2021.98
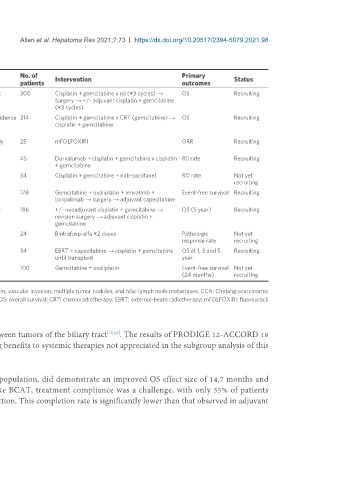
Table 4. Neoadjuvant and downstaging clinical trials in progress
Trial No. of Primary
Trial ID/name Location Tumor site Resectability status Intervention Status
type patients outcomes
NCT03673072; EudraCT 2017- Germany Phase GBC, CCA Incidental diagnosis post 300 Cisplatin + gemcitabine v nil (×3 cycles) → OS Recruiting
[56]
004444-38 (GAIN) III cholecystectomy surgery → +/- adjuvant cisplatin + gemcitabine
(×3 cycles)
CTRI/2016/08/007199; India Phase GBC Unresectable without evidence 314 Cisplatin + gemcitabine v CRT (gemcitabine) → OS Recruiting
NCT02867865 II-III of distant metastases cisplatin + gemcitabine
[57]
(POLCAGB)
NCT03603834 Thailand Phase CCA Resectable OR potentially 25 mFOLFOXIRI ORR Recruiting
II resectable
NCT04308174 (DEBATE) Korea Phase CCA, GBC Resectable 45 Durvalumab + cisplatin + gemcitabine v cisplatin R0 rate Recruiting
II + gemcitabine
NCT04546828 Korea Phase iCCA with high risk Resectable 34 Cisplatin + gemcitabine + nab-paclitaxel RO rate Not yet
II recurrence features recruiting
NCT04669496 China iCCA with high risk Resectable 178 Gemcitabine + oxaliplatin + lenvatinib + Event-free survival Recruiting
recurrence features toripalimab → surgery → adjuvant capecitabine
NCT04559139 USA Phase Incidental GBC Incidental diagnosis post 186 +/- neoadjuvant cisplatin + gemcitabine → OS (5 year) Recruiting
II-III cholecystectomy revision surgery → adjuvant cisplatin +
gemcitabine
NCT04727541 Germany Phase CCA, GBC Resectable 24 Bintrafusp-alfa ×2 doses Pathologic Not yet
II response rate recruiting
NCT04378023 Spain Phase pCCA Unresectable 34 EBRT + capecitabine → cisplatin + gemcitabine OS at 1, 3 and 5 Recruiting
IV until transplant year
NCT04523402 China Phase iCCA with high-risk Resectable 100 Gemcitabine + oxaliplatin Event-free survival Not yet
II LN metastases (24 months) recruiting
As per clinicaltrials.gov and clinicaltrialsregister.eu on July 5 2021. High risk LN features include tumor > 5 cm, vascular invasion, multiple tumor nodules, and hilar lymph node metastases. CCA: Cholangiocarcinoma;
GBC: gallbladder carcinoma; iCCA: intrahepatic cholangiocarcinoma; pCCA: perihilar cholangiocarcinoma; OS: overall survival; CRT: chemoradiotherapy; EBRT: external-beam radiotherapy; mFOLFOXIRI: fluorouracil
+ oxaliplatin + irinotecan; ORR: overall response rate; LN: lymph node.
strategy was negative. Significant clinical and genetic heterogenicity exists between tumors of the biliary tract [15,24] . The results of PRODIGE 12-ACCORD 18
may reflect this heterogenicity across biliary tract cancer subsites with differing benefits to systemic therapies not appreciated in the subgroup analysis of this
smaller trial.
BILCAP, while not achieving its primary outcome in the intention-to-treat population, did demonstrate an improved OS effect size of 14.7 months and
statistically significantly improved survival in the per-protocol analysis. Like BCAT, treatment compliance was a challenge, with only 55% of patients
completing the proposed eight cycles and 46% requiring at least one dose reduction. This completion rate is significantly lower than that observed in adjuvant