Page 28 - Read Online
P. 28
Page 8 of 15 Allen et al. Hepatoma Res 2021;7:73 https://dx.doi.org/10.20517/2394-5079.2021.98
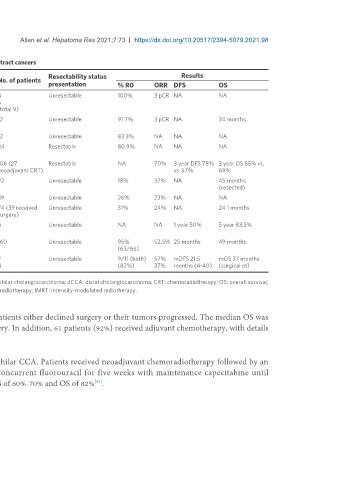
Table 3. Selected completed clinical trials of neoadjuvant or downstaging chemotherapy in biliary tract cancers
Resectability status Results
Study author Year Study type Study arms Tumor site No. of patients
presentation % R0 ORR DFS OS
[51]
McMasters et al. 1997 Prospective (non- EBRT (fluorouracil) dCCA 4 Unresectable 100% 3 pCR NA NA
randomized) pCCA 5
(total 9)
[71]
Nelson et al. 2009 Retrospective EBRT (fluorouracil) +/- pCCA, 12 Unresectable 91.7% 3 pCR NA 34 months
brachytherapy dCCA
[72]
Jung et al. 2017 Retrospective Fluorouracil/gemcitabine + EBRT pCCa 12 Unresectable 83.3% NA NA NA
[52]
Katayose et al. 2015 Prospective (non- Gemcitabine + EBRT dCCA, 24 Resectable 80.9% NA NA NA
randomized) pCCA
[73]
Kobayashi et al. 2017 Retrospective EBRT (gemcitabine) → surgery v pCCA, 106 (27 Resectable NA 70% 3 year DFS 78% 3 year OS 85% vs.
surgery dCCA, GBC neoadjuvant CRT) vs. 57% 69%
[35]
Kato et al. 2013 Retrospective Gemcitabine iCCA 22 Unresectable 18% 37% NA 45 months
(resected)
[74]
Kato et al. 2015 Retrospective Cisplatin + gemcitabine iCCA 39 Unresectable 26% 23% NA NA
[75]
Le Roy et al. 2018 Retrospective Gemcitabine + oxaliplatin iCCA 74 (39 received Unresectable 31% 24% NA 24.1 months
surgery)
[76]
Lunsford et al. 2018 Prospective case Gemcitabine → liver transplant iCCA 6 Unresectable NA NA 1 year 50% 5 year 83.3%
series
[53]
Chaudhari et al. 2018 Prospective (non- Cisplatin + gemcitabine OR GBC 160 Unresectable 95% 52.5% 25 months 49 months
randomized) gemcitabine + oxaliplatin (63/66)
[34]
Sumiyoshi et al. 2018 Retrospective IMRT (S-1) iCCA 7 Unresectable 9/11 (both) 57% mDFS 21.5 mOS 37 months
pCCA 8 (82%) 37% months (4-40) (surgical pt)
CCA: Cholangiocarcinoma; GBC: gallbladder carcinoma; iCCA: intrahepatic cholangiocarcinoma; pCCA: perihilar cholangiocarcinoma; dCCA: distal cholangiocarcinoma; CRT: chemoradiotherapy; OS: overall survival;
DFS: disease-free survival; AEs: adverse events; pCR: pathological complete response; EBRT: external-bean radiotherapy; IMRT: intensity-modulated radiotherapy.
were offered surgery, with an R0 resection achieved in 63. The remaining 94 patients either declined surgery or their tumors progressed. The median OS was
49 months, and median DFS 25 months in those who underwent curative surgery. In addition, 61 patients (92%) received adjuvant chemotherapy, with details
regarding which chemotherapy prescribed was not provided.
The “Mayo protocol” included highly selected patients with unresectable perihilar CCA. Patients received neoadjuvant chemoradiotherapy followed by an
orthotropic liver transplant. Chemoradiotherapy involved 45-55 Gy with concurrent fluorouracil for five weeks with maintenance capecitabine until
transplant . Analyses indicate improved locoregional control and a 5-year DFS of 60%-70% and OS of 82% .
[54]
[55]