Page 63 - Read Online
P. 63
Schwertheim et al. Hepatoma Res 2020;6:41 I http://dx.doi.org/10.20517/2394-5079.2020.23 Page 7 of 14
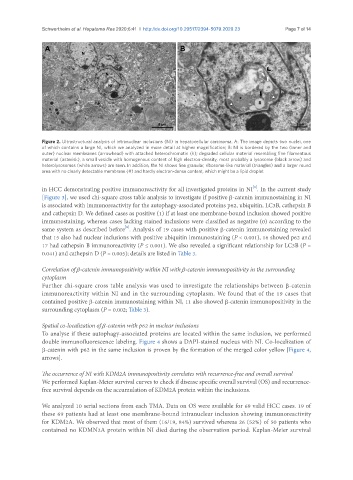
Figure 2. Ultrastructural analysis of intranuclear inclusions (NI) in hepatocellular carcinoma. A: The image depicts two nuclei, one
of which contains a large NI, which we analyzed in more detail at higher magnification; B: NI is bordered by the two (inner and
outer) nuclear membranes (arrowhead) with attached heterochromatin (h); degraded cellular material resembling fine filamentous
material (asterisk), a small vesicle with homogenous content of high electron-density, most probably a lysosome (black arrow) and
heterolysosomes (white arrows) are seen. In addition, the NI shows fine granular, ribosome-like material (triangles) and a larger round
area with no clearly detectable membrane (#) and hardly electron-dense content, which might be a lipid droplet
[6]
in HCC demonstrating positive immunoreactivity for all investigated proteins in NI . In the current study
[Figure 3], we used chi-square cross table analysis to investigate if positive β-catenin immunostaining in NI
is associated with immunoreactivity for the autophagy-associated proteins p62, ubiquitin, LC3B, cathepsin B
and cathepsin D. We defined cases as positive (1) if at least one membrane-bound inclusion showed positive
immunostaining, whereas cases lacking stained inclusions were classified as negative (0) according to the
[6]
same system as described before . Analysis of 19 cases with positive β-catenin immunostaining revealed
that 15 also had nuclear inclusions with positive ubiquitin immunostaining (P < 0.001), 16 showed p62 and
17 had cathepsin B immunoreactivity (P ≤ 0.001). We also revealed a significant relationship for LC3B (P =
0.041) and cathepsin D (P = 0.005); details are listed in Table 3.
Correlation of β-catenin immunopositivity within NI with β-catenin immunopositivity in the surrounding
cytoplasm
Further chi-square cross table analysis was used to investigate the relationships between β-catenin
immunoreactivity within NI and in the surrounding cytoplasm. We found that of the 19 cases that
contained positive β-catenin immunostaining within NI, 11 also showed β-catenin immunopositivity in the
surrounding cytoplasm (P = 0.002; Table 3).
Spatial co-localization of β-catenin with p62 in nuclear inclusions
To analyse if these autophagy-associated proteins are located within the same inclusion, we performed
double immunofluorescence labeling. Figure 4 shows a DAPI-stained nucleus with NI. Co-localization of
β-catenin with p62 in the same inclusion is proven by the formation of the merged color yellow [Figure 4,
arrows].
The occurrence of NI with KDM2A immunopositivity correlates with recurrence-free and overall survival
We performed Kaplan-Meier survival curves to check if disease specific overall survival (OS) and recurrence-
free survival depends on the accumulation of KDM2A protein within the inclusions.
We analyzed 10 serial sections from each TMA. Data on OS were available for 69 valid HCC cases. 19 of
these 69 patients had at least one membrane-bound intranuclear inclusion showing immunoreactivity
for KDM2A. We observed that most of them (16/19, 84%) survived whereas 26 (52%) of 50 patients who
contained no KDMN2A protein within NI died during the observation period. Kaplan-Meier survival