Page 9 - Read Online
P. 9
Zhuang et al. Energy Mater. 2025, 5, 500015 https://dx.doi.org/10.20517/energymater.2024.90 Page 5 of 14
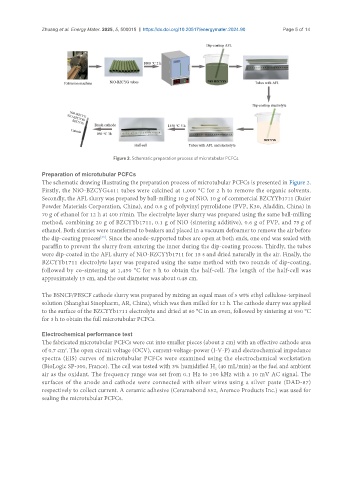
Figure 2. Schematic preparation process of microtubular PCFCs.
Preparation of microtubular PCFCs
The schematic drawing illustrating the preparation process of microtubular PCFCs is presented in Figure 2.
Firstly, the NiO-BZCYG4411 tubes were calcined at 1,000 °C for 2 h to remove the organic solvents.
Secondly, the AFL slurry was prepared by ball-milling 10 g of NiO, 10 g of commercial BZCYYb1711 (Ruier
Powder Materials Corporation, China), and 0.6 g of polyvinyl pyrrolidone (PVP, K30, Aladdin, China) in
70 g of ethanol for 12 h at 400 r/min. The electrolyte layer slurry was prepared using the same ball-milling
method, combining 20 g of BZCYYb1711, 0.1 g of NiO (sintering additive), 0.6 g of PVP, and 75 g of
ethanol. Both slurries were transferred to beakers and placed in a vacuum defoamer to remove the air before
[35]
the dip-coating process . Since the anode-supported tubes are open at both ends, one end was sealed with
paraffin to prevent the slurry from entering the inner during the dip-coating process. Thirdly, the tubes
were dip-coated in the AFL slurry of NiO-BZCYYb1711 for 15 s and dried naturally in the air. Finally, the
BZCYYb1711 electrolyte layer was prepared using the same method with two rounds of dip-coating,
followed by co-sintering at 1,450 °C for 5 h to obtain the half-cell. The length of the half-cell was
approximately 15 cm, and the out diameter was about 0.48 cm.
The BSNCF/PBSCF cathode slurry was prepared by mixing an equal mass of 5 wt% ethyl cellulose-terpineol
solution (Shanghai Sinopharm, AR, China), which was then milled for 12 h. The cathode slurry was applied
to the surface of the BZCYYb1711 electrolyte and dried at 80 °C in an oven, followed by sintering at 950 °C
for 3 h to obtain the full microtubular PCFCs.
Electrochemical performance test
The fabricated microtubular PCFCs were cut into smaller pieces (about 2 cm) with an effective cathode area
of 0.7 cm . The open circuit voltage (OCV), current-voltage-power (I-V-P) and electrochemical impedance
2
spectra (EIS) curves of microtubular PCFCs were examined using the electrochemical workstation
(BioLogic SP-300, France). The cell was tested with 3% humidified H (40 mL/min) as the fuel and ambient
2
air as the oxidant. The frequency range was set from 0.1 Hz to 100 kHz with a 10 mV AC signal. The
surfaces of the anode and cathode were connected with silver wires using a silver paste (DAD-87)
respectively to collect current. A ceramic adhesive (Ceramabond 552, Aremco Products Inc.) was used for
sealing the microtubular PCFCs.